Select a Size
About This Item
InChI
1S/C17H18FN/c18-17(16-12-7-13-19-16,14-8-3-1-4-9-14)15-10-5-2-6-11-15/h1-6,8-11,16,19H,7,12-13H2/t16-/m0/s1
SMILES string
FC([C@@H]1CCCN1)(c2ccccc2)c3ccccc3
InChI key
PGKMVPFJFKOUDA-INIZCTEOSA-N
assay
97%
optical activity
[α]/D -27°, c = 1 in chloroform
optical purity
ee: 98% (HPLC)
refractive index
n20/D 1.576 (lit.)
density
1.096 g/mL at 25 °C (lit.)
Application
- Highly efficient
- Excellent enantioselectivities
- Available in both enantiomers
- Operationally simple transformations
Catalyst for the enantioselective Weitz-Scheffer epoxidation of α,β-unsaturated aldehydes.
Condensation of this secondary amine with an aldehyde reversibly generates a β-fluoroiminium species where the fluorine atom is positioned gauche to the electropositive nitrogen centre.
Consequently, the phenyl substituents on the fluorine bearing carbon are placed in a predictable region of space. One of the groups effectively shields the upper face of the pendant iminium chain.
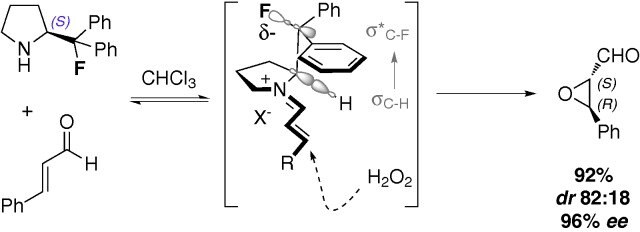 Addition of hydrogen peroxide occurs in a highly selective manner to furnish optically enriched epoxides with excellent levels of enantiocontrol (Scheme 1).
Addition of hydrogen peroxide occurs in a highly selective manner to furnish optically enriched epoxides with excellent levels of enantiocontrol (Scheme 1).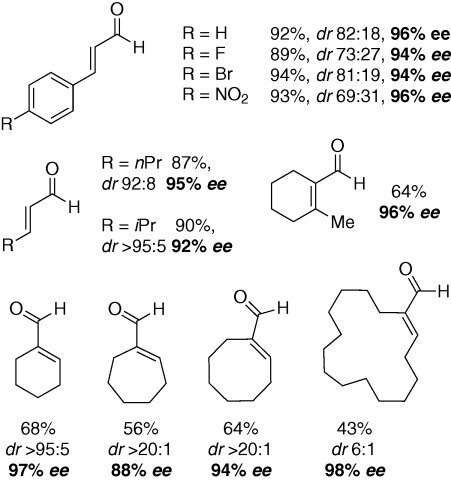 Simple α,β-disubstituted enals such as trans-cinnamaldehyde are smoothly converted to the corresponding epoxy aldehydes (up to 96% ee) as are their aliphatic analogues. The enantioselective, catalytic epoxidation of more challenging cyclic α,α,β-trisubstituted enals, and even an example of a α,α,β,β-tetrasubstituted enal, proceed with good yields and excellent enantioselectivities (up to 98% ee) (Scheme 2).
Simple α,β-disubstituted enals such as trans-cinnamaldehyde are smoothly converted to the corresponding epoxy aldehydes (up to 96% ee) as are their aliphatic analogues. The enantioselective, catalytic epoxidation of more challenging cyclic α,α,β-trisubstituted enals, and even an example of a α,α,β,β-tetrasubstituted enal, proceed with good yields and excellent enantioselectivities (up to 98% ee) (Scheme 2).Legal Information
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
10 - Combustible liquids
wgk
WGK 3
flash_point_f
147.0 °F - closed cup
flash_point_c
63.9 °C - closed cup
ppe
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Regulatory Information
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service