Custom Solutions for Cell Culture
Tailored cell culture media, feeds, supplements, buffers, and concentrates
In biomanufacturing, composition and quality of your cell culture solutions are not just a detail; they’re the foundation of your success. Each biotherapeutic process has distinct requirements, making tailored and precise customization of cell culture products crucial for both immediate results and long-term commercial viability.
Benefit from:
- Global network of redundant manufacturing sites backed by our global quality management system
- Fit-for-purpose raw materials developed and manufactured in-house, complemented by highly scrutinized sourced materials
- Industry-leading expertise and science-driven specialists to support the entire molecule life cycle and biopharma process
- Innovative solutions for process intensification and novel modalities using chemically defined media and a tailored feeding strategy
- Capabilities covering all scales and applications
- Comprehensive service offering
Explore our processes and programs to ensure consistently high product quality and performance.
Discover our unmatched capabilities for GMP cell culture products and our imMEDIAte ADVANTAGE® Services.
Learn more about our global manufacturing network and our CCM GMP certification.
Get in touch with our specialists to receive more information and discuss your needs.
Our Track Record
50+
Years supplying cell culture media for biopharmaceuticals
6
Global redundant manufacturing sites ensuring a robust supply, in region for region
100+
R&D experts for CCM applications
10,000+
Custom GMP products per year
1st
First CCM manufacturer with EXCiPACT GMP certification

Why Choose Us: Our Commitment to Quality and Excellence
In the complex world of cell culture formulations, ensuring consistency and quality is crucial for compliance. Our unwavering dedication is to deliver high-quality products and performance, underpinned by well-managed and defined supply networks, and extensive documentation.
Rely on our best-in-class quality programs including:
- Global quality management system and EXCiPACT certification: Ensuring high safety, quality, and performance of our products – and your processes. Learn more about EXCiPACT.
- Raw material and vendor management program: Detailed understanding and characterization of each component through our global program for control, management, and procurement of raw materials, including a stringent qualification program.
- Quality Services: Comprehensive R&D support for managing quality performance, including issue investigation, batch performance analysis, and raw material characterization.
- Emprove® Program: Convenient access to reliable technical, regulatory, and supply information to support your risk assessment.
- Digital tools and Data exchange: Utilizing cutting-edge technologies such as Smart Labels and eMERGE™ eData for streamlined operations and enhanced transparency.

What We Do: Empowering Innovation with Unmatched Cell Culture Capabilities
At the core of our robust in-house manufacturing capabilities is a decade-long expertise in bioprocessing, cell culture media, and a profound understanding of chemical compositions, regulatory standards, and industry best practices. Our dedication to innovation, quality, and customer satisfaction drives us to deliver tailored solutions for every phase and scale of your development and manufacturing processes.
Key capabilities:
- Custom manufacturing of cell culture solutions: Specializing in media, supplements, buffers, and concentrates tailored to meet specific application requirements.
- Custom development of cell culture media: We collaborate with you to develop customized media platforms and tailored feed solutions that meet your specific performance and quality criteria, reduce time to market, and minimize uncertainty.
- Custom compacted media: Utilizing advanced EZMix® granulation technology to deliver enhanced process efficiency and operator safety.
- In-house manufacturing of fit-for-purpose raw materials: Established raw material manufacturing program targeting key cell culture challenges and process intensification, including poloxamer cell-culture grade, low-iron impurity salts, and modified amino acids.
- Custom capabilities: Enabling small-scale pre-GMP development to large-scale GMP production, with process engineering support.
- Outstanding service offering: We provide complete solutions for our customers' needs, including extensive analytical testing capabilities, CCM stability testing studies, regulatory support with preparation and submission of DMFs, application support and technical consultation by our MSAT scientists.
Our technologies are seamlessly integrated into our CHO catalog media and feeds portfolio. Explore our highly concentrated feeds, benefit from compacted media formats, or modulate glycosylation in fed-batch processes with specially designed feeds.
Services for small-scale, pre-GMP media formulations and buffers
Our imMEDIAte ADVANTAGE® services for pre-GMP small-scale custom media, feeds, supplements, and buffers provides the quick turn-around you need to complete your development work faster – and accelerate your molecule to clinic sooner. To support global process developers, all our imMEDIAte ADVANTAGE® centers are aligned with key elements of our GMP Quality Systems, providing expedited access to the small-volume, custom cell culture products you need.
Key benefits include:
- Qualified, same-source raw materials as our large-scale GMP facilities, with the added option of unique, non-qualified raw materials for your experiments.
- Scalable systems that ensure reproducible product quality and performance.
- Master formulation management, batch management, and controlled process procedures by our trained team.
- Formulation assessment on manufacturing feasibility and product documentation incl. certificate of analysis and safety data sheet.
- Standard packaging ensuring quick delivery, product integrity, and compatibility with large-scale manufacturing.
- Development scale batch sizes: 1 - 200 L liquid / 0.5 - 20 kg dry powder.
Compatibility to Full-scale GMP Manufacturing
imMEDIAte ADVANTAGE® liquid products feature the same formulation, mixing, solubility, and filtration attributes as our large-scale GMP products. For highly concentrated liquids, our experienced raw material R&D team supports product setup with feasibility studies.
For our imMEDIAte ADVANTAGE® dry-powder media products we apply the same milling and blending controls as for our large-scale GMP products to ensure reproducible product quality and performance, while particle size, flowability, and bulk density attributes are carefully controlled to prevent caking or clumping. Products arrive ready to use, complete with hydration instructions developed either in-house or with your input.
Key Features of our imMEDIAte ADVANTAGE® Services
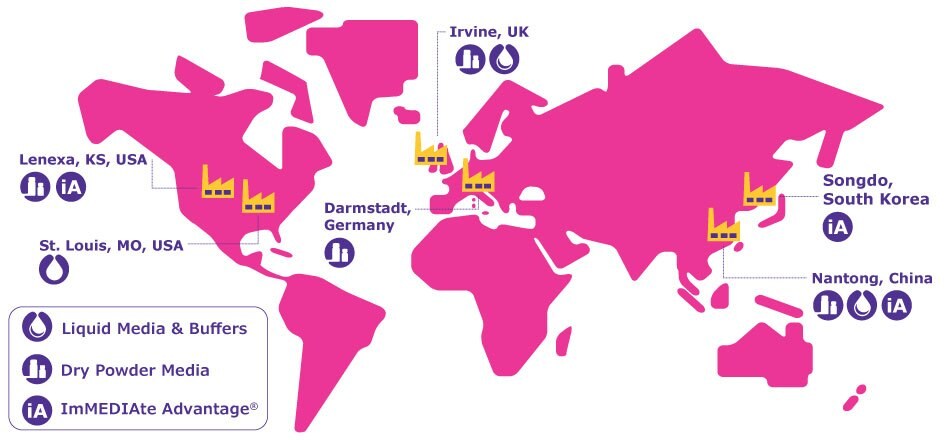
With a strong focus on product quality, site-to-site comparability, and continuous investments, we deliver world-class cell culture solutions worldwide and support our customers' business continuity.
Our strong global presence is founded upon:
- A global network of state-of-the-art facilities and dedicated expert teams
- Site-to-site comparability ensuring a robust supply
- Global quality management system
- EXCiPACT certification
- In region for region approach
- Global regulatory and technical support
Explore the distinct capabilities of each site
Prioritizing Manufacturing Excellence and Customer Collaboration
We continuously invest in and expand our manufacturing sites to meet evolving regulatory standards, fulfill the demands of our global customer base, and ensure that we are at the forefront of CCM manufacturing best practices. As of 2024, recent and ongoing investments include an expansion of our key sites in Lenexa, KS, USA and Nantong, China, as well as the establishment of new Bioprocessing Production Centers in Songdo and Daejeon, South Korea.
Complementing our manufacturing sites, we provide application support, process optimization guidance and trainings in our M Lab™ Collaboration Centers. Use the unique opportunity to connect with our experts – in-person or virtually – and benefit from the extensive experience of more than 300 scientific experts.
Establishing a Purpose-Built Cell Culture Media GMP and Certification Scheme
Historically, there had been no regulations nor a GMP certification specifically for cell culture media products, despite these materials being an integral part of the production process for many therapeutics. To address this gap, our organization collaborated with EXCiPACT leadership and other industry experts to author GMP standards for these essential materials. The result of this initiative is the application of the EXCiPACT GMP excipient standard to pharmaceutical auxiliary materials (PAMs), which include cell culture media products.
This EXCiPACT GMP standard, including the application of the EXCiPACT PAM standard together with ISO9001:2015, now defines the GMP quality system at all our current and future cell culture media product manufacturing facilities.
Related Resources
- Brochure: Custom Media Development Services
We partner with you to develop custom media solutions to meet your specific performance and quality criteria, reduce time to market, and minimize risks and uncertainty.
- White Paper: Leading the Edge of Innovation into Cell Culture Media Quality
In this white paper, we highlight the breadth of programs implemented across our organization to safeguard the quality of the cell culture media that are used in our customer processes – from some of the world’s highest grossing biologics to those produced on a patient-by-patient basis such as breakthrough cell therapies.
- Flyer: imMEDIAte ADVANTAGE® Small Volume Custom Media
Our imMEDIAte ADVANTAGE® service for pre-GMP, small scale custom media, feeds, supplements and buffers is one example of our support. This expedited service provides you the competitive advantage to complete your development work quicker and accelerate your molecule to market sooner.
- Technical Bulletin: Irvine to Lenexa Site Comparability Study
The Comparability Study was performed to demonstrate the manufacturing processes and equipment used to produce cell culture media at the Irvine, Scotland (Irvine) site are comparable to those used to produce media product at the Lenexa, Kansas (Lenexa) site.
- Flyer: Trace Elements - Data for Decisions
The role of trace elements and their impact on protein and product quality is well documented. As such, trace elements by formulation addition is critical.
- Flyer: Media Stability & Testing Services
Our Media Stability and Testing Services are two ways we can help you achieve your project timelines by letting us share your workload.
Related Technical Articles
- Establishing the First GMP Guideline for Cell Culture Media
Cell culture media are vital for bioprocessing therapeutics. This page describes the central role of our organization in authoring purpose-built GMP for cell culture media.
- Low-Impurity Iron Sources: Case Studies in EX-CELL® Advanced Medium
Discover how low-impurity iron sources improve reliability and performance in recombinant protein production with EX-CELL® Advanced medium.
- Advancing Cell Culture: Low-Impurity Iron Sources in Cellvento® 4CHO Medium
Explore how low-impurity iron sources enhance consistency and control in recombinant protein production using Cellvento® 4CHO medium.
- Glycosylation Modulation in Fed-batch Production of Therapeutic Proteins
Enhance mAb and recombinant protein performance, efficacy, and stability with precise glycosylation modulation using the Cellvento® ModiFeed platform.
To continue reading please sign in or create an account.
Don't Have An Account?