Multiplex Analysis of Low-Level Cytokines: Comparison of 384-Well vs. 96-Well High Sensitivity T Cell Panels
Chronic low-level inflammation can contribute to life-threatening diseases. High sensitivity multiplex panels allow researchers to detect low-level cytokines and study multiple cytokines at once to dive deeper into these processes. Read on to see a comparison of the performance of the 384-well assay and the 96-well assay formats for the MILLIPLEX® Human High Sensitivity T Cell Panel.
How Are Cytokines Related to Inflammation?
Cytokines are immunomodulatory polypeptides important in cell signaling, playing key roles in both adaptive and innate immune responses. They are classified based on their source or function and include interleukins (act as mediators between T cells), chemokines (responsible for T cell migration), lymphokines (produced by activated T helper (Th) cells), and myokines (produced by muscle cells). Cytokines act at the recognition, activation, and/or effector phases of an immune response to modulate the development and functional activities of T cells, B cells, and myeloid cells.
Cytokines are important mediators of inflammation, immune cell development, and immune cell activation during both normal and disease states. In addition, it is well documented that even low levels of chronic inflammation are involved in many clinical and subclinical disease states. According to the Centers for Disease Control and Prevention, low-level chronic inflammation contributes to leading causes of mortality in the United States, including cardiovascular disease, stroke, Alzheimer’s disease, diabetes, and cancer1.
Consequently, research involving cytokines is critical to achieving a deeper understanding of the immune system and the complex interactions which drive inflammatory processes that directly contribute to life-threatening diseases. This deeper understanding is facilitated by the ability to reliably detect low levels of these cytokines and having the capability to study multiple cytokines simultaneously.
High Sensitivity T Cell MILLIPLEX® Multiplex Panels
Based on Luminex® xMAP® technology, there are two MILLIPLEX® assays with identical analytes in two formats: a 384- well format and a 96-well format. The MILLIPLEX® 384-well Human High Sensitivity T Cell Panel (Cat. No. HSTC384-28K) and the 96-well version, the MILLIPLEX® Human High Sensitivity T Cell Panel (Cat. No. HSTCMAG-28SK) are both 21-plex multiplexed assay kits for simultaneously detecting cytokines significant to Th1, Th2, and Th17 cells. Both of these panels are customizable to enable the user to choose any number of analytes within the panels to meet their specific research needs. In addition, both panels are available in a premixed-bead format as a 21-plex kit. The 96-well version is also available as a fixed 13-plex premixed kit.
Comparing 384-Well and 96-Well Kit Protocols
A comparison of the protocols for the 384-well and 96-well kits is shown in Table 1. There are minor differences in the overall workflow, primarily attributable to the 384-well format (e.g., a FLEXMAP 3D® or xMAP® INTELLIFLEX instrument is required, and a plate washer, such as the BioTek® MultiFlo™ FX Automated Washer is recommended). A 384-well plate washer assures the best results. For further instructions, please refer to the kit protocols.
For serum samples, the blood was allowed to clot for 30 minutes prior to centrifugation for 10 minutes at 1000 x g. The serum was removed and either assayed immediately or stored at -20 °C. Plasma samples, with EDTA anticoagulant, were centrifuged at 1000 x g within 30 minutes of blood collection. Plasma was removed and assayed immediately, or stored at -20 °C. Frozen samples were thawed completely, vortexed, and centrifuged prior to use, to remove particulates. Neat samples were added directly into the assay plate. Samples were obtained from BioIVT, Westbury, NY.
Quantitative Multiplex Analysis Results
During the development, verification, and quality control of all kits, rigorous performance studies are conducted, including identifying the appropriate standard curve range, cross reactivity, recovery, precision, and sensitivity. The data presented below shows that the 384-well kit performs comparably, and in some instances superior, to the popular 96-well format kit.
Standard Curves
The standard curves of the MILLIPLEX® 384- and 96-well Human High Sensitivity T Cell Panels are shown in Figure 1a and Figure 1b, respectively. Both assay formats show a broad linear range with the same standard curve ranges (in pg/mL) for each analyte.
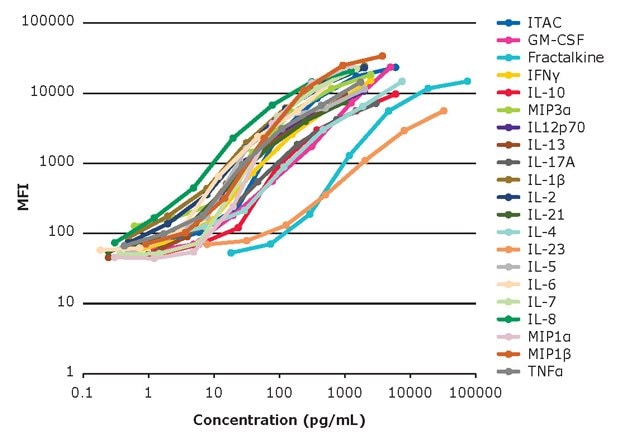
Figure 1a.Standard curves for 384-well kit.

Figure 1b.Standard curves for 96-well kit.
To get a closer look at individual analyte standard curves side-by-side, Figure 2 shows a selection of the 384-well and the 96-well analytes, plotted on the same graph.
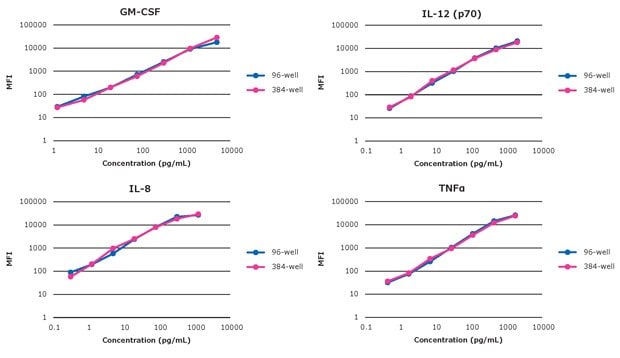
Figure 2.Select individual analyte standard curve comparisons (384-well and 96-well).
Cross Reactivity
Potential analyte cross reactivity within the assays was investigated with a single standard cross reactivity test. Each individual standard was tested in the presence of multiplexed beads and detection antibodies. All standards had less than five percent cross reactivity with the other assays. The cross reactivity data of the 384-well format was comparable to that of the 96-well format kit (data not shown).
Assay Accuracy and Recovery
Assay accuracy was determined as the percentage of the observed concentration of a known amount of standard spiked into the serum matrix. The percent recoveries were between 79% and 108% for all assays. The assay accuracy data for the 384-well format was comparable to that of the 96-well format kit, detailed in Table 2.
Precision
Intra-assay precision (%CV) was determined to be less than or equal to 10% for all analytes, examining data from eight duplicates of the standard controls. Inter-assay precision (%CV) was determined to be less than or equal to 25% for all analytes, examining data from eleven independent studies, each with duplicates of the standard controls. The inter- and intra-assay precision data for the 384-well format were comparable to that of the 96-well format kit (Table 3).
Assay Sensitivity Comparisons – 384-Well vs. 96-Well Kits
Based on the minimum detectable concentration (minDC)+2SD (Table 4), assay sensitivity for most assays was less than 1 pg/mL. The standard curves show a broad linear range of detection for all of the analytes in the panels (Figure 1a and 1b).
Sample Detection
Initial sample testing compared the percent sample detection between the 384-well and the 96-well formats. Eight healthy donor serum samples and eight healthy donor plasma samples were tested, totaling 16 samples to test the sensitivity limits of the assays. Low-level inflammation is linked to numerous clinical and subclinical diseases, but finding assays that can detect low levels of cytokines in apparently healthy individuals is a challenge. Both kits were analyzed to determine how well the panels can detect low levels of cytokines in healthy donor serum and plasma.
Both of the kit formats detected analytes at a similar frequency. Table 5 shows the number of detectable samples (analyte by analyte), while Table 6 shows the percentage of samples detectable in the 384-well versus the 96-well format.
Concerning sample correlation, Figure 3 shows high sample correlation between the 384-well and the 96-well kits for select analytes.
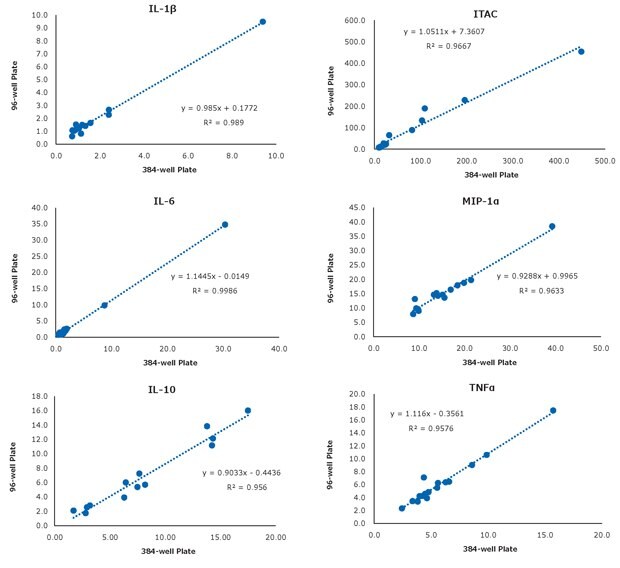
Figure 3.Select sample correlation per analyte between the 384-well and the 96-well kits (n=16 serum and plasma samples).
384-Well Quadrant Performance
The standard values obtained for specific analytes were examined, between the four quadrants of the 384-well plate. Consistent values were found for the low and high concentration analyte controls (quality controls 1 and 2, QC1 and QC2) (Figure 4).

Figure 4.Control values for analytes in the 384-well kit across four quadrants.
Benefits of the 384-Well Kit
Low-level chronic inflammation is involved in many clinical and subclinical disease states. Consequently, investigating low levels of cytokine expression will broaden our understanding of the immune system and immune cell-mediated inflammatory processes. Our MILLIPLEX® 384-well Human High Sensitivity T Cell Panel provides researchers with benefits like an analytically verified assay, superior performance, and allows for high throughput of up to 182 samples in duplicate in comparison to the 96-well version, which allows up to 38 samples in duplicate, with a standard curve and quality controls in duplicate.
This kit not only enables researchers to study low-level cytokine expression but also to quantify multiple cytokine secretion levels simultaneously, in a biologically relevant context. Extensive testing shows that the 384-well format produces comparable results to our well-known 96-well kit. Researchers with access to a FLEXMAP 3D® or xMAP® INTELLIFLEX system can use the MILLIPLEX® 384-well kit to increase throughput to approximately 17,000 data points in a single day.
Related Products
Explore our 384-well and 96-well MILLIPLEX® Human High Sensitivity T Cell Panel options.
For Research Use Only. Not For Use In Diagnostic Procedures.
References
To continue reading please sign in or create an account.
Don't Have An Account?