Multiplexing in Veterinary Medicine and Animal Health Research
Research in veterinary medicine and animal health is crucial to the protection of both animal and human health, and the advancement of science. Explore the benefits of using biomarker detection multiplex immunoassays, such as MILLIPLEX® multiplex assays, for companion and agricultural animal research.
Section Overview
- Benefits of Using Biomarker Detection Multiplex Immunoassays in Animal Research
- MILLIPLEX® Multiplex Assays for Veterinary Medicine and Animal Health Research
- Examples of Cytokine Multiplex Analysis for Agricultural Animal Research
- Examples of Pituitary Hormone and Cytokine Analysis for Companion Animal Research
- Customer Interview
- Related Vet Med Multiplex Assays
- Highlighted Publications
Benefits of Using Biomarker Detection Multiplex Immunoassays in Animal Research
Animal health research encompasses many different areas including veterinary medicine and companion and agricultural animal studies. When multiplexing with biomarker detection multiplex immunoassays, researchers can save valuable time, money, and sample volume while dramatically increasing the number of data points generated from a single assay.
MILLIPLEX® Multiplex Assays for Veterinary Medicine and Animal Health Research
MILLIPLEX® multiplex assays, based on Luminex® xMAP® technology, enable scientists studying veterinary medicine, animal health, and animal models, as well as human health, to understand complex biological systems and processes.
MILLIPLEX® Companion and Agricultural Animal kits can analyze the following animal species:
Agricultural Animals |
Companion Animals |
These highly verified assays help save time and sample volume while producing the highest quality data (Table 1).
Examples of Cytokine Multiplex Analysis for Agricultural Animal Research
View examples of ovine, chicken, and bovine cytokine multiplex assays being used in agricultural animal research below.
Ovine
The MILLIPLEX® Ovine Cytokine/Chemokine Panel 1 is the first multiplex panel designed to analyze your choice of up to 14 ovine cytokines within the same sample. See examples of data using this panel in Figures 1 and 2.

Figure 1.Ovine PBMC (BioIVT, Hicksville, NY) were stimulated for 48 hours with LPS or Concanavalin A (Con-A) or left unstimulated. Cell supernatants were collected and assayed according to the protocol in the MILLIPLEX® Ovine Cytokine/Chemokine Panel 1 (n=3, mean). The analyte IL-8 reached saturation on the standard curve for this sample group.
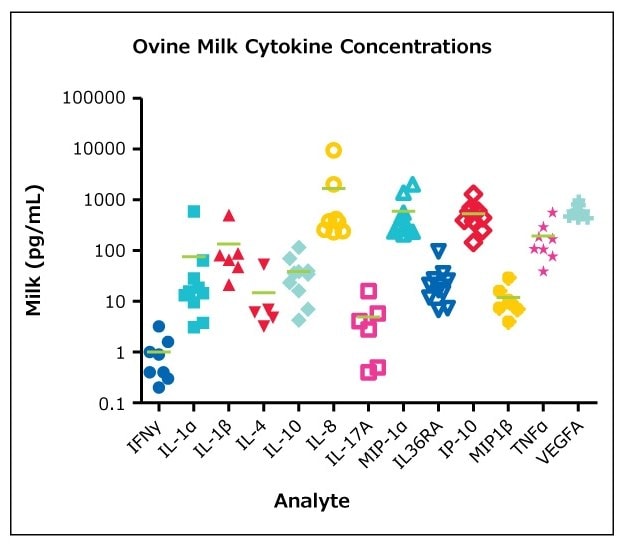
Figure 2.MILLIPLEX® ovine milk samples (BioIVT, Hicksville, NY) were assayed according to the protocol in the Ovine Cytokine/Chemokine Panel 1 (n=10, mean).
Chicken
The MILLIPLEX® Chicken Cytokine/Chemokine Panel 1 is the first multiplex panel designed to analyze your choice of up to 12 chicken cytokines within the same sample. See example data using this panel below (Figure 3).
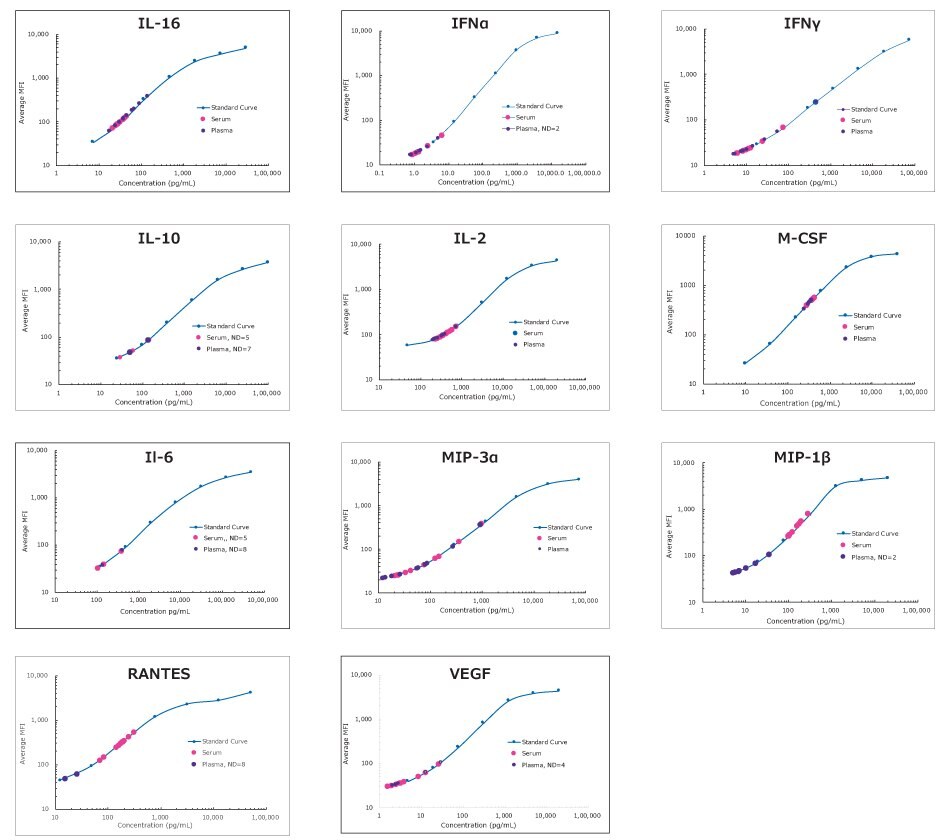
Figure 3.Normal healthy New Hampshire chicken plasma and serum samples (n=8 each) were sourced commercially and assayed according to the overnight protocol of the MILLIPLEX® Chicken Cytokine/Chemokine Panel 1. “ND=n” indicates the number of samples for which the analyte was not detected in the assay. The analyte IL-21 was not detected in these samples, however, it is expected that certain disease/inflammation states will show IL-21 values in assay.
Bovine
The MILLIPLEX® Bovine Cytokine/Chemokine Panel 1 is the first multiplex panel designed to analyze up to 15 bovine cytokines within the same sample. Figures 4 and 5 show examples of analyte data from two sample types.
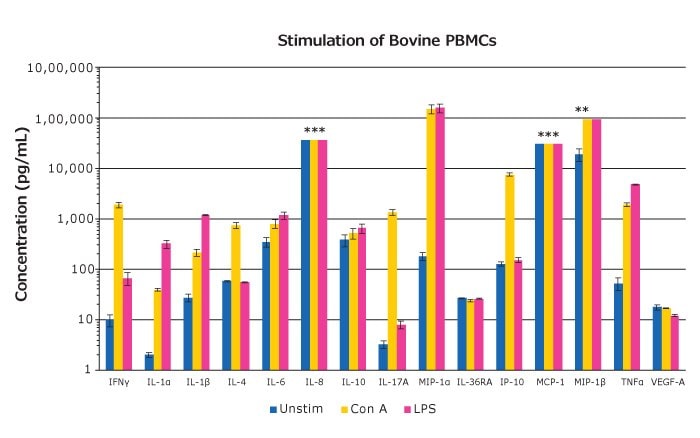
Figure 4. Bovine PBMCs (BioIVT, Hicksville, NY) were treated with LPS or Concanavalin A (Con A) for 48 hours, after which, cell-free samples were collected and assayed with the MILLIPLEX® Bovine Cytokine/Chemokine Panel 1 (n=3 mean ± SEM). *Notes saturation on the standard curve for these sample groups.
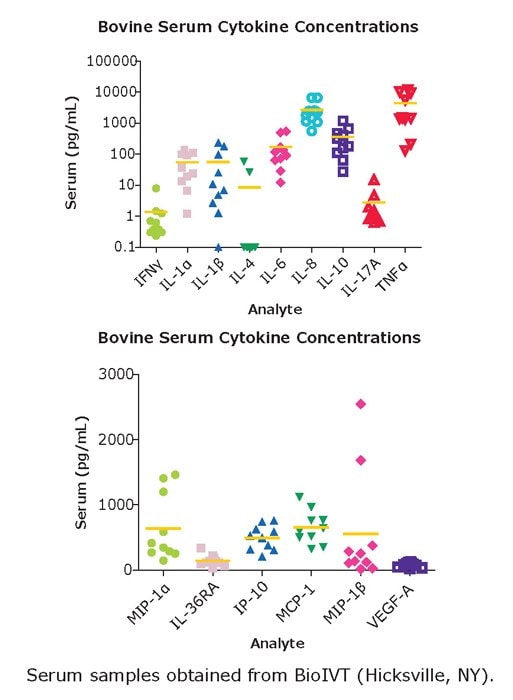
Figure 5.Serum samples were obtained from BioIVT (Hicksville, NY). Samples were assayed according to protocol in the MILLIPLEX® Bovine Cytokine/Chemokine Panel 1.
Acute phase proteins (APPs) can serve as key indicators of animal welfare and stress. The acute phase response, a systemic reaction triggered by tissue injury, infection, inflammation, trauma, or stress, results in rapid changes in APP levels in biological fluids. In cattle, APPs play a crucial role in combating infections and limiting tissue damage. Haptoglobin and Fibrinogen are two critical APP biomarkers in cattle. The MILLIPLEX® Bovine Acute Phase Protein Panel can quantitatively measure the levels of both Haptoglobin and Fibrinogen in a single sample, streamlining the process of APP biomarker analysis and offering a more comprehensive tool for assessing bovine health (Table 2 and Figure 6).
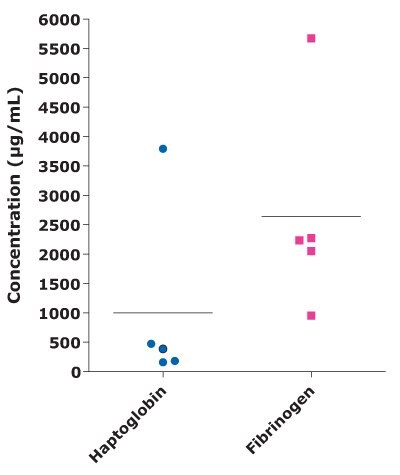
Figure 6.Bovine milk sample concentration for Haptoglobin and Fibrinogen in animals with mastitis.
Equine
Inflammation and immune cell response are key to maintaining homeostasis. The more we understand the mechanisms of disease, the more we discover the role that inflammation plays in the etiology of disease. This is as true for horses as it is for humans. Both experience immunological diseases, such as infectious diseases, osteoarthritis, and respiratory diseases, as well as neurologic, metabolic, and cardiovascular diseases and cancer. The MILLIPLEX® Equine Cytokine/Chemokine Panel is a 23-plex kit used for the simultaneous analysis of analytes in equine serum, plasma, and cell culture supernatants or tissue/ cell extracts (Table 3).
Porcine
The MILLIPLEX® Porcine Cytokine/Chemokine Panel allows for simultaneous quantification of 13 analytes in a configurable or fixed premix format (Table 4).
Acute phase proteins (APPs) constitute an integral part of the innate immune response, exhibiting significant alterations in their serum/plasma concentrations in response to inflammation, infection, or trauma. This group of proteins includes haptoglobin and C-reactive protein (CRP), which play roles in immune response and are frequently studied as biomarkers of systemic inflammation and disease. The MILLIPLEX® Porcine Acute Phase Protein Panel is a 2-plex kit for the simultaneous quantification CRP and Haptoglobin in porcine samples (Figure 7).

Figure 7.Representative standard curves with sample values from commercially sourced Yorkshire pig serum and plasma (n=10) and Minipig serum and plasma (n=8). ND=n indicates the number of samples that were below the assay limit of detection.
Examples of Pituitary Hormone and Cytokine Analysis Multiplex Analysis for Companion Animal Research
Canine
View an example of canine pituitary hormone multiplex assays being used in companion animal research below.
Quantitate canine pituitary hormones in serum, plasma, and cell/tissue culture samples of up to six analytes with the MILLIPLEX® Canine Pituitary Expanded Panel. See example analyte data in Figure 8.
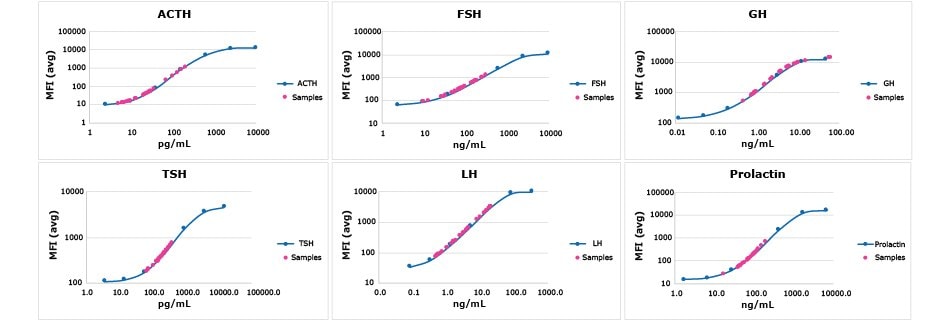
Figure 8.Commercially sourced normal canine serum (n=22) and plasma (n=27) samples were assayed according to protocol using the MILLIPLEX® Canine Pituitary Expanded Panel. Magenta circles show where each sample fell upon the indicated analyte standard curve.
Feline
Feline inflammatory markers are of interest in a variety of diseases, including sepsis, septic shock, mycobacteriosis, inflammatory oral disease, neoplastic disease, allergy, and asthma. The MILLIPLEX® Feline Cytokine/Chemokine Panel comes as a convenient fixed premix for the simultaneous analysis of 19 immune factors (Figure 9).
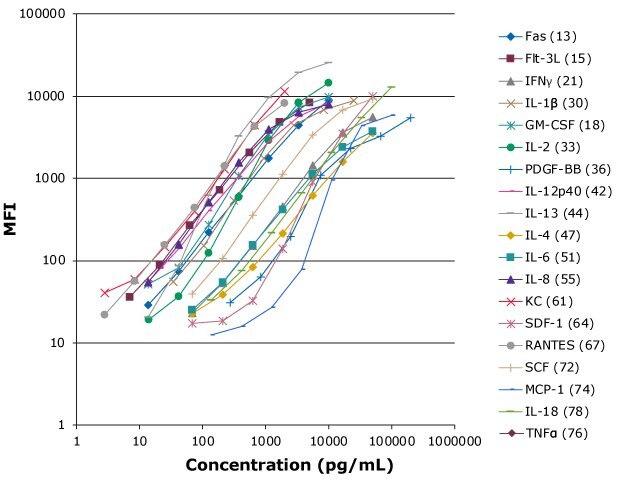
Figure 9.Representative standard curve ranges for 19 feline cytokines/chemokines.
Customer Interview
Q&A on Agricultural Research Using A Bovine Cytokine/Chemokine Multiplex Panel
Read our interview with Dr. Kyle McLean, PAS, Assistant Professor in Ruminant Reproduction, from the Department of Animal Science at the University of Tennessee Institute of Agriculture to see how MILLIPLEX® panels helped advance his agricultural animal research.
Briefly describe your current research.
My research focuses on ruminant reproduction with a particular focus on the uterine environment, placental development, and fetal programming in early gestation.
Specifically, how do cows fit into your research?
Cows are the focus of my research.
Why did you choose cows?
It has been and will always be the focus of my research due to the economic importance and biomedical potential of cattle.
How does using the MILLIPLEX® Bovine Cytokine/Chemokine Panel 1 help your research and/or workflow?
This panel has allowed me to quantify more cytokines with less sample and more quickly than anything else. It has also allowed us to develop a profile for the uterine environment.
What other great work is being done in your laboratory?
We are also establishing the amino acid profile of the uterine environment as well as looking into the impacts of nutrition on the composition of seminal plasma in bulls.
If you could solve any challenge in research, what would it be?
Understand the mechanisms behind the establishment of pregnancy and establish the nutrient requirements of both mother and embryo during pregnancy.
Do you have any advice for scientists starting out in your field?
Don’t be afraid to ask the hard questions and think outside the box.
Ovine
Chicken
Bovine
Equine
Porcine
Canine
Feline
Multi-Species
If you cannot find the assay you are looking for, we also offer custom assay services to help you multiplex it your way and develop the right assay for your research.
For Research Use Only. Not For Use In Diagnostic Procedures.
Agricultural Animals
Ovine
Chicken
Bovine
Equine
Porcine
Companion Animals
Canine
Feline
To continue reading please sign in or create an account.
Don't Have An Account?