HPLC Method for the Separation of Nine Key Silymarin Components of Milk Thistle
Introduction
Silymarin, derived from the milk thistle plant, has been used as a natural remedy for the treatment of a number of liver diseases as well as in the protection of the liver from potential toxins. More recently, silymarin has been connected to antitumor promoting activity.1 It is therefore of interest to have available analytical methods for the analysis of silymarin components in various matrices.
The silymarin complex consists of several closely related, biologically active components. The objective of this study was to investigate several modern stationary phase chemistries for optimal selectivity toward the development of an efficient method for the analysis of silymarin. The method would optimally provide baseline resolution for nine of the known components of silymarin in less than 15 minutes.
Initial screening experiments were used to select a single combination of stationary phase and organic modifier that demonstrates the greatest potential for the separation. The information from subsequent optimization experiments was processed with simulation software to predict an optimized set of conditions. Finally the predicted conditions were confirmed using the analysis of an herbal supplement obtained from a local drug store.
Experimental
The screening protocol used five Ascentis Express stationary phases, including C18, C8, RP-amide, phenyl-hexyl and pentafluorophenylpropyl (F5). Gradient elution with two distinct organic modifiers was performed. Injections of a standard containing nine of the known constituents of silymarin (Figure 1) were made under each condition.
The HPLC screening method used gradient elution from 5 to 95% of either methanol or acetonitrile. The aqueous component of the mobile phase was 0.1 % formic acid (pH 2.6).
In order to optimize the separation, separate chromatographic analyses at two different gradient slopes and two temperatures were performed. These four runs were then processed using ACD LC-Simulator software (Toronto, ON, Canada) and a predictive model was used to develop conditions that would provide baseline resolution of all peaks in less than 15 minutes. The conditions were then confirmed using both standards and a commercially available milk thistle herbal supplement.

Figure 1. Structures of known biologically active silymarin component.
Results and Discussion
Using a simple peak counting approach, the combination the C18 stationary phase along with methanol as the organic modifier resulted in the most visible peaks with nine. A representative chromatogram is shown in Figure 2. Other combinations of stationary phase and organic modifier produced between six and eight peak responses.
Four separate chromatographic runs using the C18 stationary phase and methanol modifier were conducted where the gradient slopes were varied (5% and 10% ramp) at two different temperatures (30 °C and 60 °C). The data was then analyzed using ACD LC-Simulator to predict the most suitable optimized conditions. Comparison to the experimental data shown in Figure 3 confirms good agreement between the predicted (not shown) and actual chromatography. Because the suggested conditions ran to just 45% organic, an additional step to 100% organic was included to elute potential hydrophobic components from a natural sample.
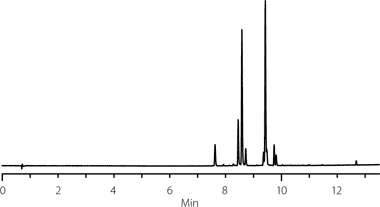
Figure 2.Initial separation of silymarin components using C18 stationary phase and methanol as the organic modifier.
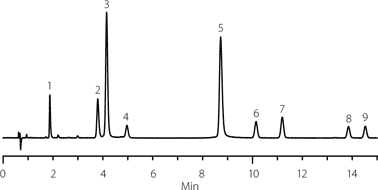
Figure 3. Experimental confirmation of predicted optimized conditions.
Finally an herbal supplement labeled as containing milk thistle along with dandelion, fennel and licorice was extracted using water:ethanol 50:50, v/v and analyzed. As shown in Figure 4, all 9 of the targeted components could be observed in the herbal supplement material.
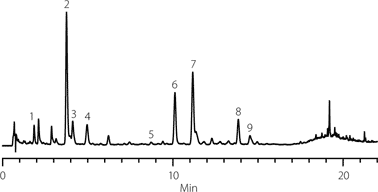
Figure 4. Identification of silymarin components from a commercial natural supplement.
Conclusions
Stationary phase screening at the onset of method development, especially when dealing with a complex set of analytes, provides a facile means of analytical method development. As shown in the study, a few short experiments, coupled with powerful prediction software provided chromatographic conditions suitable for the analysis biologically active milk thistle compoenents. The developed conditions should prove useful in natural supplement, raw material and/or biological monitoring of silymarin complex components.
References
To continue reading please sign in or create an account.
Don't Have An Account?