Extractables Studies of Single-Use Equipment Immediate Identification and Quantification of Unknown Extractables by GC/MS with a Certified Reference Material Mix for Extractables and Leachables

Introduction
The manufacture of active pharmaceutical ingredients, drug substances, or drug products generally involves several production steps, during which the process stream may inadvertently pass along components of the production unit consisting of polymeric materials. Caused by this direct contact, an accumulation of so-called leachables in the process stream could occur, potentially compromising the safety and quality of the desired product.
One such example of components made up of polymeric materials within the production process are single-use systems (SUS). Manufacturers of single-use equipment are interested in studies on their materials to provide an overview of possible leachables in the end users’ products. Extractables data are generated in a laboratory under a variety of possible production conditions, thereby providing valuable information on what could potentially occur during the process.
Leachables: Chemical compounds that migrate into a drug formulation from any product contact material (e.g., single-use systems) as a result of direct contact under typical process or storage conditions; Leachables may affect the toxicity or efficiency of the drug product
Extractables: Chemical compounds that are extracted from any product material usually under extreme conditions (harsh solvents, exaggerated time and temperature); an Extractables profile represents a worst-case Leachables profile
Single-use Systems (SUS): Usually polymeric, disposable equipment for bioprocessing used for the manufacturing of pharmaceuticals
Advantages: Flexibility, no need for cleaning validation, low investment, no cross contamination
Examples of SUS: Bioreactors, disposable filters or tubing
According to BPOG (BioPhorum Operations Group) guidelines1 and USP 665 guidelines for polymeric components and systems2 (draft version), investigations regarding extractables should be performed using various solvents and incubation times and analyzed using a variety of analytical methods applied to the extracts. One of these analytical techniques is gas chromatography-mass spectrometry (GC-MS), which is used for the determination of semi- volatile extractables such as additives, impurities, polymer components, degradation products, or extraction solvent-material interaction products.
If the exact composition of the polymeric material used to make the single-use equipment is unknown, a non-targeted analysis is crucial. To accelerate the identification, we have launched a certified reference material (CRM) mixture of the most common extractables detected via GC/MS (Figure 1) as well as neat reference materials of the components. Using these materials, not only can these 14 components be identified, but also their quantification with traceability to NIST SRM is possible. In the following, an application is described for the Extractables and Leachables Screening Standard for GC mix, for the identification and quantification of the main extractables present within an extractable study of a filter.
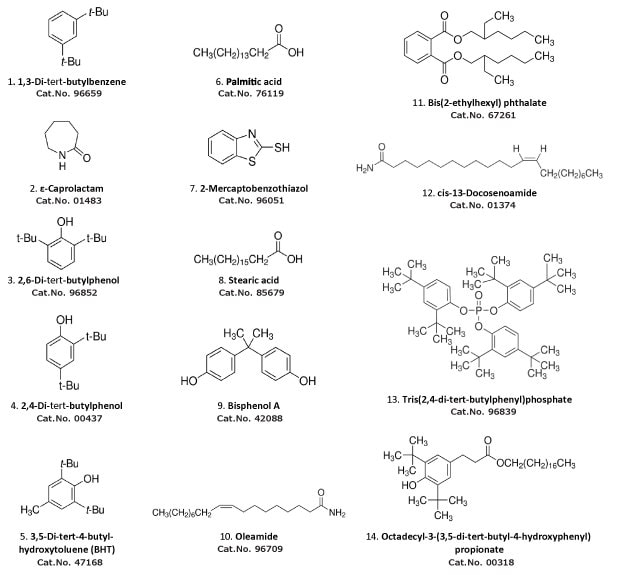
Figure 1.Components of the Extractables and Leachables Screening Standard for GC Cat. No. 01829; in elution order of below method. Concentration is 50 mg/L in tert-butyl methyl ether for each component. For all the analytes, individual neat reference materials are also available under the catalog numbers listed.
GC/MS Method for Extractables Testing
The applied instrument parameters for an extractable study on single-use equipment are summarized in Table 1. According to the BPOG protocol1, the separation was performed on a Supelco® SLB®-5ms Capillary Column. A representative sample taken after seven days of extraction at 40 °C under orbital rotation with WFI (water for injection) was prepared and further processed by liquid-liquid extraction (LLE) using dichloromethane as extraction solvent and p-terphenyl-d14 as LLE extraction standard. The sample was fortified with phenanthrene-d10 as internal standard prior to analysis. The sample and the standard mix ran in one sequence.
Results & Discussion
The chromatogram of the Extractables and Leachables Screening Standard for GC is shown in Figure 2. All 14 reference compounds were detected and almost completely separated. Due to mass spectrometric detection, coeluting components could be identified by their extracted ion chromatogram. By matching retention time and m/z ratio, ε-caprolactam was identified as the main extractable during the extraction of the single-use filter (Figure 3). A quantitative analysis against the caprolactam peak within the Extractables and Leachables Screening Standard for GC could be performed based on the extracted ion chromatograms.
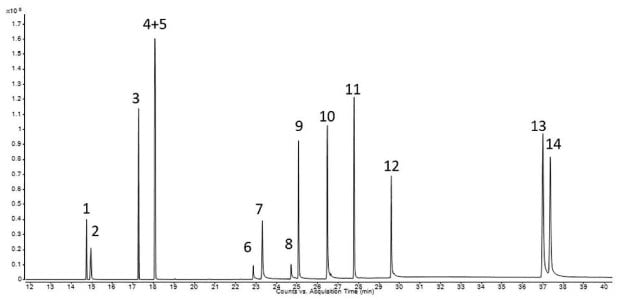
Figure 2.Extractables and Leachables Screening Standard for GC, 50 mg/L in tert-butyl methyl ether (Peak IDs - Table 2).
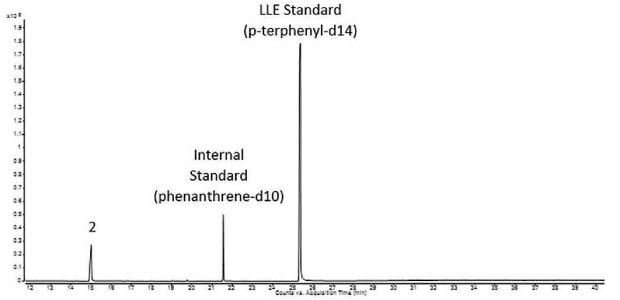
Figure 3.Representative sample of a single-use equipment extraction (Peak IDs - Table 2).
Conclusion
The example shown demonstrates the applicability and value of the Extractables and Leachables Screening Standard for GC for the reliable identification and quantification of the most common extractables resulting from single-use equipment.
To see our complete portfolio of reference materials for extractable and leachable testing, visit us at SigmaAldrich.com/extractablesandleachables.
Related Products
References
To continue reading please sign in or create an account.
Don't Have An Account?