Why Is Flow Through Polishing Needed in Downstream Processing?
As today’s monoclonal antibody (mAb) manufacturers continue their quest for improved productivity, robustness, flexibility and efficiency, intensified processing is increasingly being evaluated. Upstream intensification in seed train and production and harvest steps are complemented by next-generation manufacturing process analytical technology (PAT) and automation and control capabilities, which together are transforming traditional approaches to biomanufacturing. However, to fully realize the benefits of upstream productivity, downstream capture and polishing steps need to evolve.
As the first step in downstream purification, protein A capture chromatography has long been recognized as a bottleneck in purification. Rather than the typical single large protein A column used in batch processing, intensified downstream processing that incorporates multicolumn capture chromatography achieves higher loadings and productivity with smaller volumes of protein A resin and lower costs.1 Following capture and viral inactivation, downstream polishing removes process and product impurities and contributes to overall process viral clearance targets. As these steps represent ~25% of production costs, any gains from process intensification will positively impact process economics.
For optimal processing efficiency, downstream polishing steps need to match the output of the upstream process. But there are multiple ways to meet these demands: the capacity of individual unit operations could be increased, processing time of these operations decreased, parallel processing strategies could be used or some combination of these.
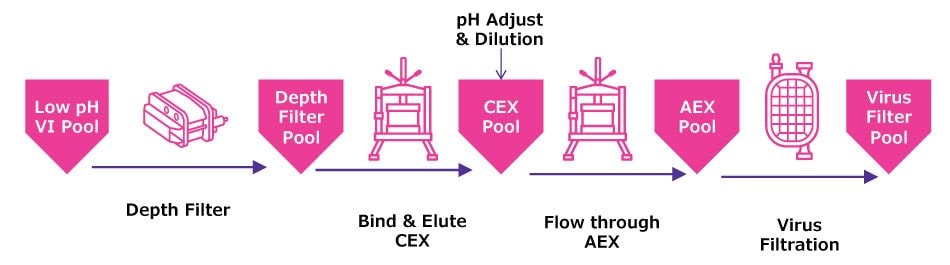
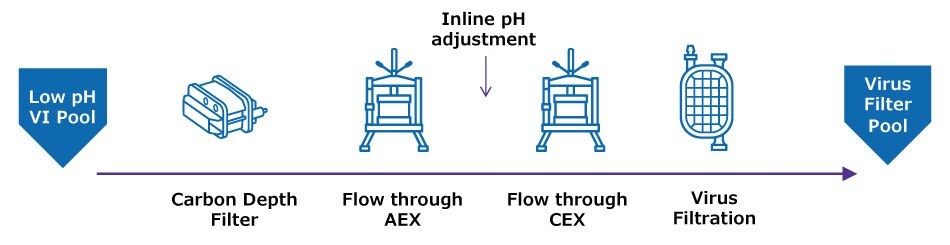
Figure 1 illustrates typical downstream polishing operations in both batch and flow through polishing operations. Batch operations typically include bind elute steps, where the molecule of interest interacts with the chromatography media while impurities flow through; conditions are then adjusted to elute the molecule from the media. By contrast, in flow through operations impurities bind to the media while the molecule of interest flows through. Both batch and flow through polishing templates include:
- Depth filtration following viral inactivation to reduce HCP, particulates, and turbidity.
- Multiple chromatography steps: usually anion exchange (AEX) chromatography to reduce host cell protein (HCP), residual nucleic acids and leached protein A, and cation exchange (CEX) chromatography to reduce protein aggregates. Multimodal chromatography may be implemented to provide additional selectivity for impurity removal. The sequential order of AEX and CEX steps is process-dependent.
- Virus filtration for viral clearance, which may include a prefiltration step to maximize virus filter capacity.
Technologies for Efficient Downstream Purification
The diversity and complexity of mAbs means no one set of technologies will likely meet all purification needs. Various polishing technologies can be considered, dependent on the benefits they deliver to each process. For any single molecule one or, more likely, multiple purification technologies will be needed to meet the molecule purity specifications. Each technology offers multiple product options, each with specific operating windows; this means buffer or pH adjustments may be necessary to transition from one step to the next. Planning any downstream polishing operation should consider adjustments and intermediate holds as part of the holistic process and incorporate these into any assessments of productivity.
Bioburden Control
Any downstream polishing operation should also consider bioburden control measures, including bioburden reduction or sterilizing grade filtration. Filters designed to deliver high capacity with plugging feed streams are preferred; examples include Milligard® PES bioburden reduction filters or Millipore Express® SHC sterilizing-grade filters. For more stringent bioburden control, while potentially reducing environmental control requirements for manufacturing, closed processing technologies could also be considered.2
Depth Filtration
Depth filters efficiently remove different types of impurities by size exclusion, entrapment or adsorption and operate in flow flow through mode under constant flow. They are available with different media materials in single or multiple layers and different pore sizes to meet various clarification needs. Following viral inactivation at low pH and neutralization, depth filtration is often performed to reduce aggregate and particulate levels before chromatographic separation. This post-viral inactivation clarification is considered part of downstream polishing as it improves separation efficiency in chromatography steps.
Filters containing diatomaceous earth offer highly selective removal of large molecules, protein aggregates and negatively charged impurities such as nucleic acid and HCP. However, as the media is a natural product the supply chain is more complex and contains certain contaminants not found in synthetic media. Filters containing synthetic depth filtration media, such as Millistak+® HC Pro filters are often preferred due to the absence of beta glucans, more consistent performance, improved capacity and lower flush volumes.
Filters containing activated carbon are often recommended for polishing applications following viral inactivation. Their highly porous structure, high surface area and unique adsorptive characteristics make them particularly effective for binding small molecules such as protein fragments and phenolic or aromatic compounds, found in mammalian cell culture feeds. Low molecular weight impurities are typically removed during bind/elute chromatography operations, but in fully continuous bioprocesses, which rely on flow through purification, this removal can be more challenging. Activated carbon technologies, such as Millistak+®CR40 pod filters, operate in flow through mode across a wide range of conditions and effectively remove low molecular weight HCP impurities regardless of charge.
Anion Exchange Chromatography
Anion exchange (AEX) chromatography is a key component of any downstream polishing process and is typically performed in flow through mode using either a resin or membrane technology. Negatively charged impurities such as certain HCP, DNA, endotoxin, and many viruses bind to the chromatography medium and the positively charged target molecule flows through.
With most AEX resins the resin loading capacity is 100 – 250 g mAb/L resin, which results in large columns and long processing times. In addition, before AEX separation, the conductivity of the process fluid must also be adjusted to < 10 mS/cm and levels of phosphate reduced for efficient impurity removal. However, the efficiency of AEX steps using resins such as Eshmuno® Q resin can be improved by preconcentrating the process fluid using single pass tangential flow filtration (SPTFF ). SPTFF concentrates both the mAb and impurities and improves isotherm binding, which increases impurity adsorption and resin loading. Conductivity adjustments for the AEX step can be made either before or after SPTFF: adjustments before concentration will be maintained through the SPTFF step so the concentrated molecule can be directly purified on AEX; adjusting after SPTFF reduces dilution buffer requirements and may improve tank sizes, flow rates and facility fit. Importantly, the smaller volume of more concentrated fluid following SPTFF reduces AEX column volumes, resin loading times and means less time is needed for column regeneration and sanitization. Combined, implementing SPTFF improves AEX step productivity to the point it is economically advantageous, even with the addition of an extra processing step.
Membrane technologies are an attractive single-use alternative to chromatography resins and are ideally suited to meet the needs of intensified downstream processes. Natrix® Q chromatography membrane is an anion exchange membrane technology that provides fast flow rates, high dynamic binding capacity, mass loading capacities of >10 kg/L, and a wide range of operating conditions, including tolerance to salt and phosphate, two key limitations of resin technologies and other non-hydrogel based membrane chromatography devices.
Cation Exchange Chromatography
CEX chromatography is often used in downstream polishing to separate the mAb monomer from undesirable protein aggregates and HCPs. This step is usually performed in bind/elute mode and, dependent on the process, is performed either before or after AEX chromatography. If CEX chromatography is positioned following viral inactivation and depth filtration, usually a minor pH adjustment is required for loading; by contrast, AEX chromatography following viral inactivation requires larger pH and conductivity adjustments. However, placing CEX chromatography step downstream of the AEX chromatography step maximizes the opportunity for aggregate removal immediately before virus filtration, a step where high aggregate concentrations can negatively impact virus filter capacity.
For purification based on bind/elute CEX technologies, Eshmuno® CPX resin and Eshmuno® CMX resin offer effective separation with wide operating windows for pH and conductivity, high dynamic binding capacities for impurity removal and excellent separation of mAb monomers from aggregate impurities. Eshmuno® CPX resin is a strong CEX resin and delivers high purity at low elution volumes, while Eshmuno® CMX mixed-mode resin provides high selectivity and efficient separation of low molecular weight impurities through weak cation exchange and hydrophobic interaction properties and is better option for more complex separation needs. Eshmuno® CPX resin can also be run in flow through mode which may simplify polishing operations. However, this may come at the cost of reduced purification efficiency.
By contrast, Eshmuno® CP-FT resin offers the benefits of efficient aggregate removal but in a flow through frontal chromatography mode of operation which enables 10× higher loading capacities than traditional bind/elute CEX chromatography resins. Higher loading capacity means smaller volumes of resin and smaller columns with less time dedicated to column regeneration and sanitization. These benefits result in significant cost savings, improved productivity and an improved sustainability profile.
Natrix® CH chromatography membrane utilises CEX and hydrophobic interaction chromatography (HIC) ligands, offers very high capacity and enables efficient separation of aggregates and HCP impurities in either flow through or bind/elute modes. Membrane technologies operate at fast flow rates and offer an attractive single-use option for manufacturers looking to improve efficiency and minimize time spent on column regeneration and maintenance.
Virus Filtration
Virus filtration is a dedicated virus reduction step towards the end of downstream polishing and is a critical component of process viral safety.
A primary goal of polishing purification is to minimize aggregate concentration and maximize throughput and loading on the virus filter. Protein aggregate impurities in the process fluid prematurely foul small pore virus filters, resulting in oversized filtration areas to achieve the required capacity. Downstream polishing operations must deliver robust purification performance to reliably meet the throughput capacity and loading defined for the specific molecule on the virus filter.
The Viresolve® Pro Solution includes a virus filter and prefiltration solutions to provide the highest levels of virus retention and productivity across a broad range of operating conditions. The Viresolve® Pro Device (virus filter) provides excellent performance in terms of virus retention and loading, under the very high flux conditions associated with traditional batch processing. Similar performance has also been reported under low flux conditions more typical of intensified continuous processes.3 Achieving loading targets often requires implementation of an adsorptive virus prefilter immediately upstream of the virus filter to remove trace levels of aggregates that remain after polishing.
Benefits of Transitioning to a Flow Through Polishing Platform
The pressure on biomanufacturers to maximize efficiency and reduce costs has resulted in upstream intensification and significant productivity gains. This however, presents significant challenges in the downstream process, as contaminant loads are higher and could be a bottleneck in the process.4 Improvements in downstream can be achieved by optimizing traditional operations, but these are still long duration, labor intensive processes, with intermediate hold tanks and large columns requiring large quantities of buffer. While the efficiency benefits of flow through polishing have long been reported, the transition from batch to flow through processing has been slow. 5,6
Flow through polishing integrates purification operations without the need for large intermediate hold tanks, requiring only much smaller surge vessels. By implementing technologies that deliver efficient separation in flow through mode, the molecule can be purified, and impurities can be removed, with significant savings in buffers, process duration and cost of goods. Process modelling analysis, quantified the benefits of flow through polishing:
- Cost of Goods: Reduced by 43%
- Reduced buffer volume
- Less resin
- Fewer buffer and product hold bags
- Process time: Reduced by 72%
- Higher productivity
- More batches/year
- Buffer volume: Reduced by 87%
- Significantly lower buffer volumes
- Lower material costs
- Smaller footprint
But implementing flow through polishing means thinking about the steps holistically as the operational conditions for any one step will have an impact on the separation and purification efficiency of others. The case studies in the following section highlight various flow through polishing strategies with different molecules.
Different Strategies for Flow Through Polishing
In batch processing, AEX chromatography binds negatively charged impurities and the molecule flows through. If needed, CEX polishing chromatography medium binds positively charged mAb and impurities flow through; then the purified molecule is eluted from the medium. In intensified flow through polishing, the absence of bind/elute CEX purification means that multiple flow through polishing steps, each with orthogonal mechanisms of separation, are needed to selectively remove impurities from the process fluid. For process robustness, both size and charge should be considered, Table 1.
Establishing a polishing train usually involves consideration of the operating windows of the various technologies to maximize yield and impurity removal. Broad operating windows are often preferred, so different technologies can operate synergistically with minimal solution adjustments.
Case studies highlight performance of a flow through polishing purification strategy and illustrate the benefits of a holistic approach. You can read our whitepapers for different examples.
The case study summarized below describes a limited data set from a more comprehensive study that benchmarked performance of activated carbon, Eshmuno® Q and Eshmuno® CP-FT resins against commercially available alternative products. Each technology was evaluated individually and then as part of an integrated process, with activated carbon, followed by AEX chromatography, followed by CEX chromatography. Loading on the Eshmuno® Q and Eshmuno® CP-FT columns were 2000 g/L; full details of the study are described in the whitepaper, but results demonstrated:
- Activated carbon was a main driver of HCP removal and enabled increased loading on Eshmuno® Q resin.
- Results were independent of conductivity, and only slightly influenced by pH; conditions were selected to minimize pH and conductivity adjustment before AEX.
- Eshmuno® Q resin improved the loading capacity of Eshmuno® CP-FT resin
- Operating pH was selected to maximize HCP removal; higher pH improved viral clearance.
- Eshmuno® CP-FT resin, was a main driver of HCP and aggregate removal and increased the loading capacity on the Viresolve® Pro virus filter.
- Operating pH was selected to maximize HCP and aggregate removal. Lower conductivity improved aggregate clearance.
The results of the integrated flow through polishing train with these technologies are summarized in Table 4.
Studies compared the filtration performance of this purification train to a more typical filtration train comprising the Viresolve® Pro Shield prefilter upstream of the Viresolve® Pro Device filter. The protein feed had HCP level of 4070 ppm and contained ~5% aggregates; Eshmuno® CP-FT resin was loaded to 1,000 mg/mL and the pressure for virus filter could not exceed 30 PSI. Figure 2 illustrates the significant benefits of higher loading on the virus filter when the feed is purified over Eshmuno® CP-FT resin immediately upstream of the virus filter.
mAb1 - High Aggregates
Viresolve® Pro Experiment w/mAb1 with ~5% Aggregates Loading vs. Pressure
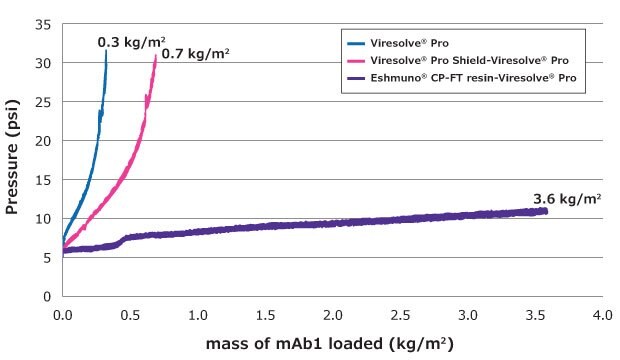
Figure 2.Impact of pretreatment on virus filter loading
Conclusions
As bind/elute chromatography steps are productivity bottlenecks, the move to a fully flow through polishing strategy is a prerequisite for intensified and eventually continuous bioprocess purification operations.
This approach is not without its challenges; the absence of hold tanks necessitates a holistic approach to process development, validation and ultimately the process control in GMP manufacturing.
Disruptive technologies, such as frontal chromatography using Eshmuno® CP-FT resin, will greatly expedite the evolution towards intensification, by reducing process footprint, process time and process cost. The benefits of full bioprocess intensification can be realized today with current technologies and are increasingly being adopted in processes worldwide.
Related Products
References
To continue reading please sign in or create an account.
Don't Have An Account?