Angiogenesis Assays
Angiogenesis Tube Formation Assays
Microfluidic Angiogenesis Assays
Endothelial Adhesion, Invasion and Migration Assays
Scratch Wound Healing Assays
Endothelial Cell Characterization
Endothelial Cells and Culture Media
Angiogenesis is the process of generating new capillary blood vessels and is a fundamental component of a number of normal (reproduction and wound healing) and pathological processes including tumor growth and metastasis. Abnormal blood vessel growth can be the underlying cause of many deadly and debilitating diseases including cancer, cardiovascular disease, stroke, and diabetic and age-related blindness.
In tumors, dysregulated signaling and hypoxic conditions lead to sustained uncontrolled angiogenesis, a necessary component of tumor growth and metastasis. Chronic inflammation mechanisms, such as the production of reactive oxygen species (ROS) and secretion of pro-inflammatory cytokines, can also foster angiogenesis in tumor progression. Angiogenic signaling in tumors is similar to normal angiogenesis, mediated by soluble growth factors, membrane-bound receptors, and cell-cell and cell-matrix interactions. Such signaling regulates cell migration, which is vital to angiogenesis. However, there are multiple differences between tumor angiogenesis and normal blood vessel formation. Tumor endothelial cells proliferate faster than non-tumor endothelial cells. Tumor vasculature differs from normal vasculature in morphology, enhanced leakiness, and structural abnormalities. Finally, tumor vessels are often not capable of transporting oxygen to and removing waste products from all of the tumor tissues, resulting in frequent tumor cell necrosis.
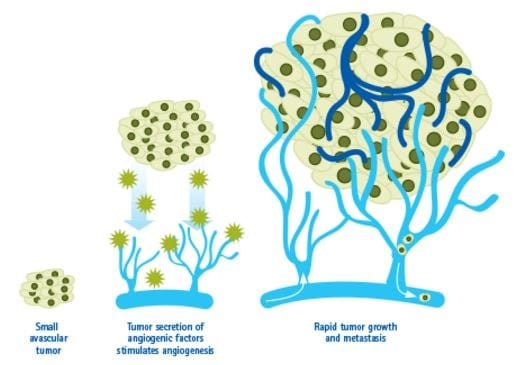
Figure 1. Angiogenesis in Cancer.Tumors can stimulate nearby normal cells to produce angiogenesis signaling molecules resulting in new blood vessels formation. These new vessels in turn supply growing tumors with oxygen and nutrients, allowing the cancer cells to invade nearby tissue, to move throughout the body, and to form new colonies of cancer cells, called metastases.
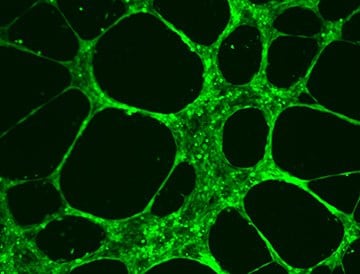
Figure 2.HUVEC Angiogenesis Assay.
HUVEC cells form extensive tubular vascular networks after incubation of 6-10 hours at 37 °C on ECM Matrix provided in the In Vitro Angiogenesis Assay Kit (Product No. ECM625).
Microfluidic Angiogenesis Assays
Studying how compounds affect angiogenesis, either to promote or inhibit new capillary tube formation can lead to therapies affecting wound healing, tissue regeneration, cardiovascular disease, stroke, tumor progression, and more. The Millicell μ-Angiogenesis activation and inhibition kits provide a powerful, quantitative platform for live cell monitoring of changes in tubule formation with unprecedented optical resolution.
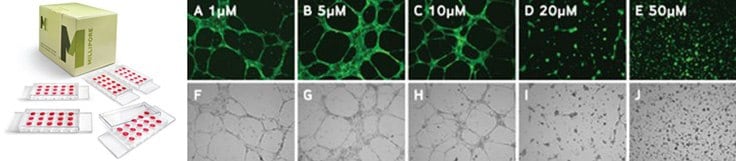
Figure 3.Increasing concentrations of sulforaphane resulted in decreases in both mean tube length and mean number of branch points as shown below in bright field and calcein-AM micrographs of HUVEC cells.
Endothelial Adhesion, Invasion, and Migration Assays
Endothelial cells invade through the basement membrane to form sprouting blood vessels within tumors. The invasion process consists of the secretion of matrix metalloproteases (MMPs) to degrade basement membrane, the activation of endothelial cells, and the migration of cells across the basement membrane. The understanding of invasion is important for studying the mechanism of angiogenesis in injured tissue as well as in disease such as cancer.
QCM™ Boyden chamber cell invasion assays enable convenient and sensitive quantification of in vitro cell invasion, vascular permeability, adhesion, and migration through an endothelial cell layer.
Scratch Wound Healing Assays
The scratch assay is a popular method for the study of cell migration and angiogenesis. Briefly, endothelial cells are grown to confluence and a wound is introduced by clearing an area of the monolayer using a pipet tip, needle or cell scraper. Cell filling of the cleared space initially occurs by migration, though cells in the cleared area will eventually also proliferate. Scaling up this technique has proved challenging, however, making biochemical analysis of the molecular events mediating wound repair difficult. The Cell Comb™ scratch assay addresses the need for a simple tool able to create multiple scratch wounds in a higher-throughput manner.
Read our Application Note in Nature

Figure 4.Scratch Wound Healing Assays
Endothelial Cell Characterization
Endothelial Cells and Culture Media
Primary cells maintain physiological relevance because they are derived directly from living tissue and thus find increasing use in life science research and pharmaceutical drug. We are pleased to offer primary cells from Cell Applications Inc. including endothelial cells and optimized growth media from microvascular, aortic, coronary, pulmonary, lung and umbilical cord (HUVECs) tissues.
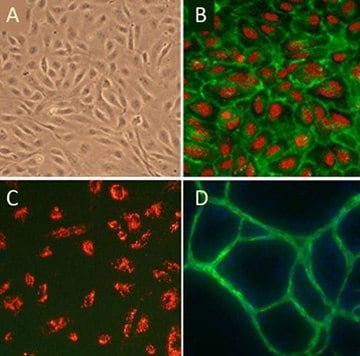
Figure 5. (A)Cobblestone morphology of Human Umbilical Vein Endothelial Cells, HUVEC; (B) HUVEC immunolabeled with VEGFR2 antibodies (green); (C) HUVEC stained with DiI-Ac-LDL (red), the acetylated apoprotein specifically recognized and endocytosed by endothelial cells; (D) HUVEC form vessel-like CD31/PECAM positive (green) structures when cultured with Human Dermal Fibroblasts in the presence of VEGF.
References
To continue reading please sign in or create an account.
Don't Have An Account?