The Role of High-Purity Metal Salts in Enhancing Chemical Synthesis
Introduction
Catalysts play a central role in shaping the course and efficiency of organic synthesis. Their ability to influence reactivity, selectivity, and sustainability has led to the evolution of diverse catalytic paradigms, including transition metal catalysis, organocatalysis, photocatalysis, electrocatalysis, and biocatalysis.1,2 Among these, metal catalysis continues to be a cornerstone of modern synthetic methodology. Metal-containing compounds, including metal complexes, oxides, and simple metal salts, are widely utilized in both academic and industrial laboratories. These compounds react under homogeneous, heterogeneous, and solid-supported conditions, enabling a wide range of organic transformations. They are useful not only for speeding up reactions and increasing yields, but also for making protocols easier to use and more sustainable.²
Beyond their primary catalytic roles, metal salts perform a variety of additional functions in organic synthesis. They are frequently used in reaction workups as quenching agents or to increase the solubility of reagents and intermediates. They can also act as acids, bases, phase-transfer catalysts (PTCs), electrolytes, or neutral media, and they are commonly used to convert organic molecules into salt forms for increased reactivity or isolation. Many metal salts also function as Lewis acids or bases, providing precise control over reaction pathways and selectivity.¹
Transition metal salts, in particular, have enabled important bond-forming reactions that support complex molecule construction. Palladium catalysts are commonly used in C-C bond-forming reactions such as the Suzuki-Miyaura, Heck, and Stille couplings, whereas nickel-based systems are popular in Kumada-type couplings. Copper salts are essential for C-heteroatom bond formation via Ullmann-type and Chan-Lam couplings, which provide access to C-N, C-O, and C-S linkages. Gold complexes have shown promise in hydroamination and hydroalkoxylation of alkynes, whereas iron- and cobalt catalysts have gained popularity as earth-abundant and cost-effective alternatives in oxidative cross-coupling and C-H functionalization strategies. These transformations demonstrate the catalytic versatility and synthetic potential of metal salts. Their ability to enable efficient, selective, and sustainable reactions has made them essential tools in the modern synthetic chemist's toolbox.
In this article, we examine the role of high-purity metal salts in organic synthesis reactions, discussing their benefits across a wide range of organic transformations aimed at the creation of new molecular architectures.
Figure 1.Organic reagents and transition metal catalysts.
Importance of High-purity Metal salts
High-purity salts are characterized by the presence of minimal impurities, such as heavy metals and anions like sulfate, chloride, and nitrate, which are known to disrupt organic synthesis pathways. Their exceptional purity ensures the reliability that is essential for industrial applications.3 For example, in the pharmaceutical sector, high-purity metal salts are essential in bioprocessing and the development of therapeutic compounds. Salts such as calcium chloride (499609, 202940, 429759), magnesium sulfate (203726, 940518), and iron(III) nitrate (529303, 254223) facilitate cell growth and metabolism, serving as enzyme cofactors and engaging in crucial cellular signaling. Achieving a purity level of 99.99% or higher is imperative, as even minor impurities can adversely affect cell culture performance and product quality.
Role of High-purity Salts in Different Type of reactions
In chemical synthesis, high-purity salts are crucial materials, acting as precursors for heterogeneous catalysts, active catalysts in homogeneous reactions, and vital auxiliaries. Their function in catalysis is essential for enhancing reaction efficiency, enabling high product yields, improving chemical selectivity to reduce by-products, and decreasing overall energy consumption.
Metal Salt Catalysts in Different Synthesis Reactions
Hydrogenation Reactions
The hydrogenation of various C=C bonds through H₂ cleavage and addition has been an essential technique for over a century, impacting sectors such as petrochemicals, food production, agriculture, and pharmaceuticals.4 Precious metals, including Ru, Rh, Pd, and Ir, are critical in industrial hydrogenation due to their greater effectiveness in breaking H₂ molecules and providing hydrogen to substrates.5 Recently, there has been an increasing interest in abundant and more sustainable transition metal catalysts, including iron, manganese, cobalt, copper, nickel, and magnesium. For instance, cobalt-based catalysts (Figure 2A) are being investigated for their ability to convert α-primary amino ketones into amino alcohols,6 while nickel-based catalysts (Figure 2B) have been employed to synthesize β2-amino phosphorus derivatives.7
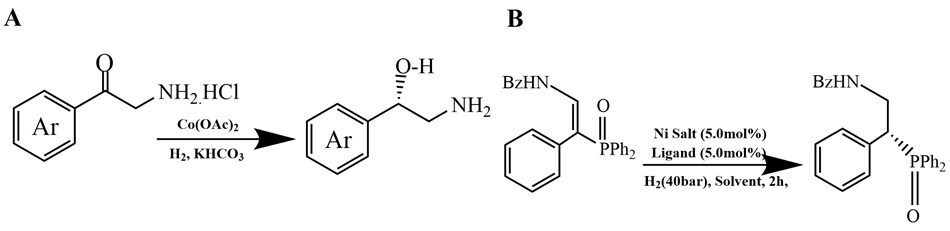
Figure 2.Hydrogenation reactions catalyzed by (A) Cobalt salts6 and (B) Nickel salts7
Cross Coupling reactions
Cross-coupling reactions using nickel and palladium catalysts are frequently employed to introduce functional groups to unsaturated compounds, such as alkenes and aromatic rings. Microencapsulated Pd(OAc)2 (520764, 379875) within polyurea serves as a cost-effective and adaptable heterogeneous catalyst for multiple phosphine-free cross-coupling reactions, including carbonylation, Heck, Suzuki, and Stille reactions, which can be conducted in both traditional solvents and supercritical media, allowing for simple and efficient separation from reaction mixtures using filtration process.8 Furthermore, Pd(OAc)2 mediated (Figure 3) C-H bond arylation using aryl-BF3K salts enables the transformation of various electron-deficient arenes under mild reaction conditions.9

Figure 3.Pd(OAc)2 encapsulated catalyst for cross coupling reactions8.
Oxidation Reactions
Transition metal salts play an important role in catalyzing the autoxidation of organic compounds, especially in the oxidation of ketones and aldehydes with molecular oxygen under mild conditions. Compounds such as Mn(NO₃)₂ (935697, 203742), Co(NO₃)₂ (203106, 935719) and Cu(NO₃)₂ (940143, 923079, 923087, 229636) show effectiveness, with manganese salts being particularly important, as they facilitate the enolization of carbonyl compounds and begin a free-radical redox chain with oxygen. While cobalt and copper salts are inert on their own, they increase the catalytic effectiveness of manganese salts and enhance the breakdown of hydroperoxides. (Figure 4)10
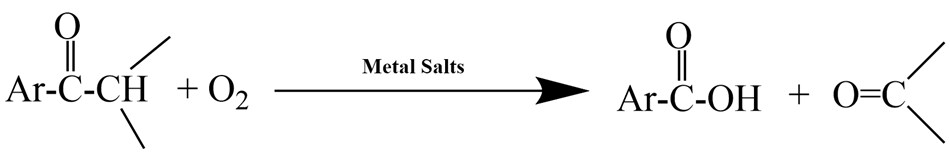
Figure 4.Transition metal salt catalyzed oxidation reaction10.
Electrochemical Organic Synthesis
The integration of homogeneous catalysis with electrochemical synthesis introduces a novel driving force for catalytic cycles, facilitating the production of reactive intermediates at electrodes and reducing dependence on external oxidants.11 Transition-metal salts prove to be efficient mediators and catalysts in electrochemical processes, like the cathodic hydrogen evolution reaction (HER), removing the need for stoichiometric oxidants in X–H bond functionalization.12-14 For example. Lin et al. made significant progress by establishing a method for the electrochemical diazidation of alkenes utilizing MnBr2.4H2O (208434, Figure 5) [E2+/0 = −1.17 V vs normal hydrogen electrode (NHE)] without requiring an additional ligand.15 Likewise, a cobalt-catalyzed electrochemical C–H alkylation was developed utilizing Co(OAC)2.4H2O as a precursor for a Co(I)/Co(III) cycle that produces intermediates at the anode. This cycle efficiently integrated with a cathodic HER, thereby eliminating the requirement for an external oxidant.16
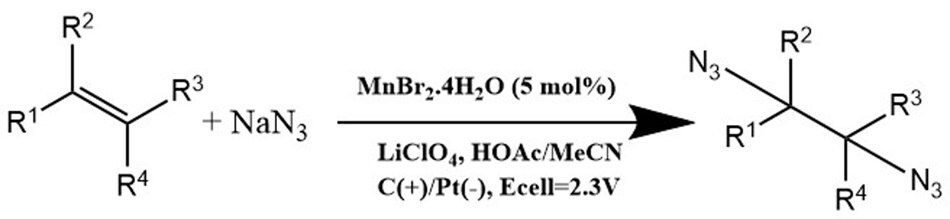
Figure 5.Alkene Diazidation reaction by transition metal salts.15
Furthermore, noble metal salts such as Cu, Ru, Rh, Pd, Ag, Re, Os, Ir, Pt, and Au exhibit low standard reduction potentials, making them more susceptible to undergoing reduction before protonic species. A potential solution to this problem is to utilize a divided cell to isolate the metal from the cathode, but this approach increases expenses and diminishes mass transfer efficiency.11 Thus, it is advantageous to perform electrochemical reactions involving these metals in undivided cells. For example, Kathiravan et al. demonstrated a Cu-catalyzed electrochemical C–H amination using Cu(OAc)2 (517453, 326755), where a 20 mol% catalyst loading facilitates the direct anodic oxidation of the Cu complex to initiate the catalytic cycle (Figure 6A). In addition, Ackermann's research group employed a 5 mol% Cu catalyst with Cu(OAc)2·H2O (229601), using a directing group to achieve alkyne annulation via a C–H alkynylation route (Figure 6B).17,18

Figure 6.Cu(OAc)2 catalyzed electrochemical C–H functionalization with chelation in an undivided cell.17,18
In addition to Cu-based catalysts, research has also shown that RhCl₃ catalyzes the electrochemical C–H/C–H coupling of benzoic acids through dehydrogenation. It has been demonstrated that RhCl₃ (940615) provides superior results compared to [Cp*RhCl₂]₂ in DMF, while a bimetallic Rh complex was utilized for C–H oxygenation reactions. On the other hand, employing a Cp*Rh complex, [Rh(OAc)₂]₂ (209058), featuring a stable dimeric configuration, the product's selectivity was influenced by varying the electricity input.19,20 Additionally, there has been extensive research into Pd-based homogeneous catalysis due to its elevated reduction potential.8,9,11 Consequently, developing techniques that take advantage of the electrochemical driving force as well as the selectivity provided by homogeneous catalysis is crucial. Innovations that improve diffusion rates and facilitate electron transfer between electrodes and catalysts, establish new mediating pathways, and create robust catalysts have the potential to significantly enhance electrochemical synthesis and catalysis. Overall, these advancements would foster progress within this field.
Metal Salts as Precursors for Heterogeneous Catalysts
The role and effectiveness of metal salts as precursors for heterogeneous catalysts are influenced by both their cation and anion.21,22 The cation provides the source of the metal or metal oxide, while the anion affects the properties of the salt during decomposition. These catalyst materials based on metal oxides are employed in various reactions, including oxidation, hydrogenation, dehydrogenation, biomass conversion, photocatalysis, and electrocatalysis, owing to their active sites related to redox and acid-base reactions.22 However, these intricate oxides are produced through different methods such as solid-state synthesis and sol-gel coprecipitation to yield highly pure materials from high-purity metal salts (Figure 7).

Figure 7.Synthesis method of heterogeneous catalyst using metal salts precursors.
Conclusion
High-purity transition metal salts are vital for organic synthesis, significantly improving the efficiency and reliability of chemical reactions. Their low impurity levels prevent contaminants from interfering with synthesis, which is particularly important in the pharmaceutical industry, where even minor impurities can impact product quality. The high purity of these salts enhances their catalytic performance, resulting in increased reaction yields, improved selectivity, and reduced formation of by-products. Acting as precursors for both homogeneous and heterogeneous catalysts, high-purity salts support more-sustainable practices, easier handling, and speeding up reaction times.
Finally, high-purity salts contribute significantly to the advancement of organic synthesis, by enabling the production of high-quality compounds across a range of applications.
References
To continue reading please sign in or create an account.
Don't Have An Account?