Sulfonyl Chlorides and Sulfonamides
Sulfonyl chlorides are often chosen as building blocks in medicinal chemistry for their ability to easily react with heterocyclic amines to create complex sulfonamides. One such report details the use of cyclopropanesulfonyl chloride and cyclopentanesulfonyl chloride as building block in the synthesis of TNF-a converting enzyme (TACE) inhibitors (Scheme 1).1 The cyclopropyl variant exhibited good selectivity for TACE over MMP-2 and -13.
Another recent communication describes the synthesis of human glucocorticoid receptor (hGR) ligands.2 This sulfonamide created from a-methyltryptamine and 2,4,5-trichlorobenzenesulfonyl chloride (Scheme 2) exhibited micromolar hGR potency. The sulfonamide moiety was also demonstrated to be crucial for effective hGR binding in the study.
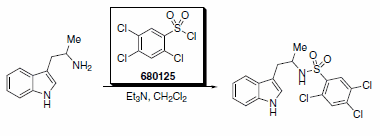
Scheme 2. Product Number: 680125
(2-Trimethylsilyl)ethanesulfonamide (SES-NH2) has proven effective at directly introducing a protected nitrogen-functionality into a molecule. One example is in the work of Bolm and Mancheño, who reported the use of SES-NH2 in the iron-catalyzed imination of sulfoxides to yield sulfoximines (Scheme 3).3

Scheme 3.
Lamaty and co-workers have prepared a series of SES-protected b-aminoesters by using SES-NH2 in an aza-Baylis-Hillman reaction. These b-aminoesters were then further elaborated to provide a series of 2,3-disubstituted pyrroles through a ring-closing metathesis protocol (Scheme 4).4
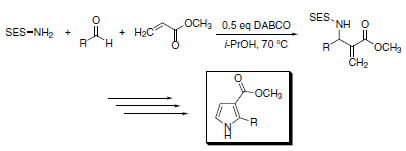
Scheme 4.
Related Materials
References
To continue reading please sign in or create an account.
Don't Have An Account?