Trimethyl(trifluoromethyl)silane (Ruppert–Prakash Reagent)
One of the most widely used reagents for nucleophilic trifluoromethylation is trimethyl(trifluoromethyl)silane, TMSCF3. The reagent has become widely referred to as the Ruppert–Prakash reagent, after Ruppert who introduced the material in 1984,1 and Prakash who is largely responsible for popularizing its use.2 The broad applicability of the TMSCF3 makes it a popular reagent in syntheses of various medicinal targets.
In one example, a series of four tri- and tetraglutamic acid and glutamine peptides were created that incorporate a trifluoromethyl ketone group.3 The β-amino alcohol synthon was synthesized in five steps where the key step utilized TMSCF3 to create the resultant trifluoromethylated silyl ether as a single diastereomer (Scheme 1). Two of the peptides in the report were shown to exhibit inhibitory activity against severe acute respiratory syndrome coronavirus protease (SARS-CoV 3CLpro).
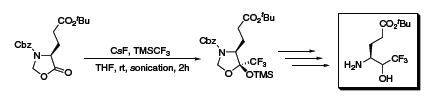
Scheme 1
Another example usesTMSCF3 in the synthesis of a non-steroidal selective androgen receptor modulator that displays excellent oral bioavailability and anabolic activity in muscle (Scheme 2).4 The compound also improved bone strength in a rat model of postmenopausal osteoporosis. Similarly, Hudson and coworkers used TMSCF3 to create a selective glucocorticoid receptor modulator that demonstrated antiproliferative activity equal to the myeloma therapeutic, dexamethasone (Scheme 3).5
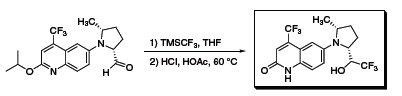
Scheme 2
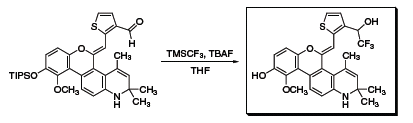
Scheme 3
Commonly, an additional fluoride source (TBAF, CsF, etc.) is required to initiate the trifluoromethylation reaction. However, a recent report from Prakash detailed the trilfuoromethylation of carbonyl compounds using a new series of catalysts which do not require additional fluoride initiators or water-free conditions (Scheme 4).6
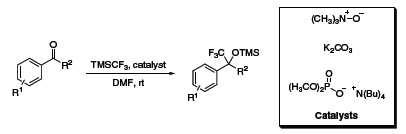
Scheme 4
Another report from Prakash demonstrated the use of TMSCF3 on N-unactivated imines to yield the corresponding trifluoromethylated amine. With a slight variation in the reaction conditions, difluoromethylated amines can also be prepared via HF elimination and reduction (Scheme 5).7
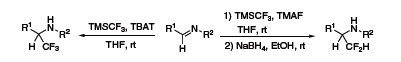
Scheme 5
Despite its wide use, stereoselective trifluoromethylation using the Ruppert–Prakash reagent is still challenging. However, several groups have made significant progress in this arena. Dieter Enders has reported diastereoselective trifluoromethylation of α-alkylated dioxanones with good yields and high diastereo- and enantiomeric excesses.8 The acetonide group is easily removed to generate the 2-trifluoromethyl-1,2,3-triols (Scheme 6).
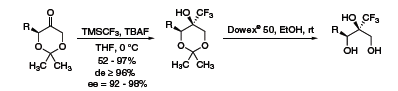
Scheme 6
Shibata, Toru, and coworkers have reported an operationally simple method for enantioselective trifluoromethylation based on the combination of the ammonium bromide of a cinchona alkaloid, 1, and tetramethylammonium fluoride (TMAF).9 The reaction proceeds in modest to excellent yields with ee’s up to 93% (Scheme 7).
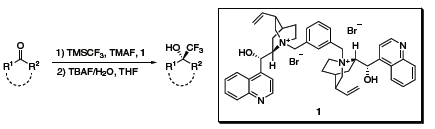
Scheme 7
To continue reading please sign in or create an account.
Don't Have An Account?