ChiPros Chiral Alcohols
Chiral alcohols form a versatile class of chiral synthons, since they can be incorporated into the API structures directly as esters or ethers. They can be starting materials for the formation of amines, amides, thiols, thioethers. In addition, after transforming the hydroxyl function into a leaving group by way of mesylation, tosylation or triflation, they can be used to form new C–C bonds.
Many manufacturing routes make use of asymmetric hydrogenation methods.2 The two most important biocatalytical processes for the formation of chiral alcohols apply lipases and dehydrogenases, respectively.1 The latter offers the advantage that only the requested enantiomer is obtained. Enzyme-catalyzed acylations using lipases, however, achieve the resolution of racemic mixtures of alcohols but with an inherent 50 percent maximum yield of the total amount of starting material. One enantiomer of the racemic mixture remains unchanged while the antipodal enantiomer is esterified (Scheme 2).
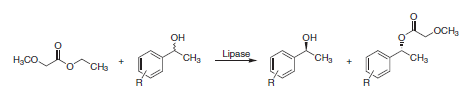
Scheme 2.Lipase-catalyzed resolution of aryl-substituted alcohols.
Thanks to a variety of commercial and proprietary enzymes at its disposal, BASF offers a wide range of aliphatic and cycloaliphatic and aryl-substituted single-enantiomer alcohols under the ChiPros brand.
Materials
References
To continue reading please sign in or create an account.
Don't Have An Account?