Chiral Vicinal Diamines for Asymmetric Synthesis
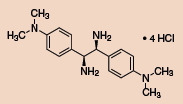
Chiral vicinal diamines are of tremendous interest to the synthetic chemist as they are found in many chiral catalysts and pharmaceuticals. Currently, there is no unified approach to making these chiral vicinal diamines, and they are often challenging to synthesize, especially if unsymmetrically substituted. Jik Chin and co-workers have recently reported some preliminary theoretical and experimental studies for converting a parent diamine (1) into other chiral vicinal diamines.1 These diamines can be used as ligands for chiral catalysts, or they can be further elaborated to produce chiral heterocyclic rings and b-lactams via ring closure.
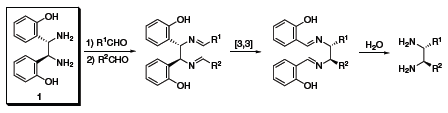
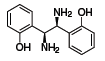
(S,S)-1 (685879)
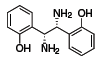
(R,R)-1 (685860)
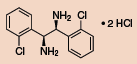
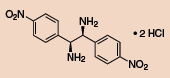
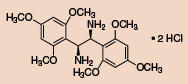
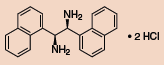
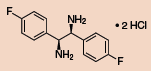
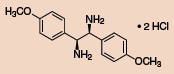
Other Chiral Vicinal Diamines
Materials
Loading
References
1.
Chin J, Mancin F, Thavarajah N, Lee D, Lough A, Chung DS. 2003. Controlling Diaza-Cope Rearrangement Reactions with Resonance-Assisted Hydrogen Bonds. J. Am. Chem. Soc.. 125(50):15276-15277. https://doi.org/10.1021/ja0387554
2.
Kim H, Kim H, Alhakimi G, Jeong EJ, Thavarajah N, Studnicki L, Koprianiuk A, Lough AJ, Suh J, Chin J. 2005. Preorganization in Highly Enantioselective Diaza-Cope Rearrangement Reaction. J. Am. Chem. Soc.. 127(47):16370-16371. https://doi.org/10.1021/ja055776k
3.
Kim H, Kim W, Lough AJ, Kim BM, Chin J. 2005. A Cobalt(III)?Salen Complex with an Axial Substituent in the Diamine Backbone: Stereoselective Recognition of Amino Alcohols. J. Am. Chem. Soc.. 127(48):16776-16777. https://doi.org/10.1021/ja0557785
Sign In To Continue
To continue reading please sign in or create an account.
Don't Have An Account?