Q-Phos
The use of electron-rich, sterically congested monodentate phosphine ligands, in conjunction with a metal complex, has become a common paradigm in transition metal-catalyzed coupling reactions. Developed by Professor John Hartwig, pentaphenyl(di-tert-butylphosphino)ferrocene (Q-Phos, Figure 1) has emerged to be a premier ligand in coupling reactions with its remarkably broad utility in a variety of C–N, C–O, and C–C bondforming reactions. Additionally, the pure ligand is exceedingly robust, being stable in air and in solution.
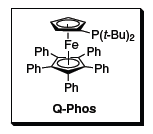
Figure 1.Chemical structure of Q-Phos.
Amination
In combination with common Pd complexes, Q-Phos is an excellent ligand for the amination of aryl chlorides and bromides of varying electronic character with both primary and secondary amines.1 Typically, the amination reactions reach completion in less than 24 hours at temperatures below 100 °C, and in certain cases, at room temperature. Generally, only small amounts of hydrodehalogenated byproducts are observed. The reaction protocol relies on either Pd(dba)2 or Pd(OAc)2 as palladium sources, and a base/solvent system of either NaOt-Bu/toluene or K3PO4/DME. As illustrated in Table 1, diarylamines rapidly couple with aryl bromides at room temperature (entry 1). Less expensive aryl chlorides also couple in high yield, although higher temperatures are required (entry 2). Similarly, alkylarylamines, dialkylamines, and anilines are viable nucleophiles for the coupling reaction (entries 3–7). In the case of primary aliphatic and benzylic amines, the amination was remarkably selective for the monoarylated product (entries 8 and 9). Finally, for some substrates, it is possible to conduct the amination at very low catalyst loadings (e.g., entry 10).
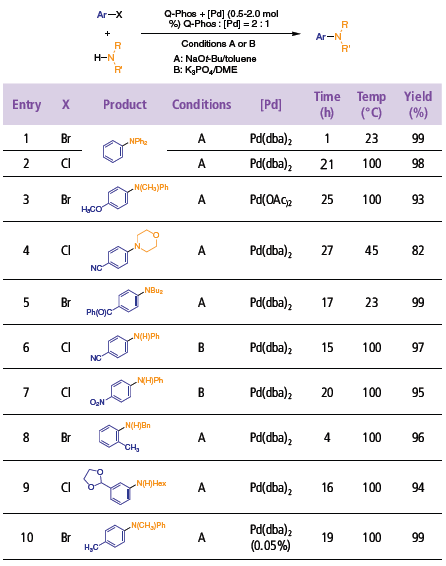
Table 1.
Etherification
Until recently, mild aromatic C–O bond-formation was exceedingly difficult to achieve. Palladium complexes of Q-Phos catalyze the mild etherification reaction of aryl halides with alkoxide, siloxide, and phenoxide partners (Table 2).1,2 Reactions with aryl bromides, or electron-poor aryl chlorides, generally proceed at room temperature. The catalyst system was also successful in the synthesis of coumarins, via intramolecular etherification.
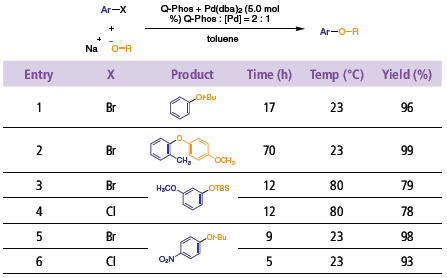
Table 2.
Suzuki-Miyaura Coupling and α-Arylation
Q-Phos are highly successful for both electron-poor and electron-rich aryl bromides and chlorides (Table 3).1 As expected, aryl bromides are more reactive, and typically Suzuki couplings with arylboronic acids proceed at room temperature (entry 3). Reactions of aryl chlorides with aryl boronic acids generally require heating for extended periods. Additionally, alkylboronic acids couple easily with aryl bromides and chlorides, without the aid of thallium or silver salts (entries 4 and 5).
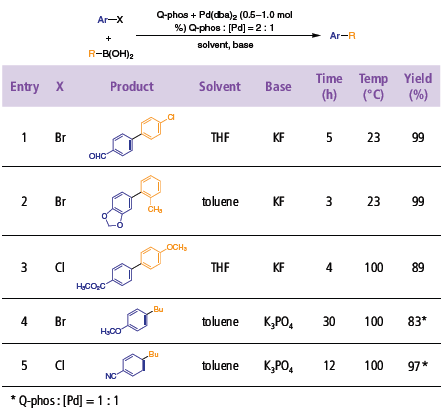
Table 3.
As illustrated in Scheme 1, Q-Phos is an effective ligand for the palladium-catalyzed α-arylation of either diethyl malonate or ethyl cyanoacetate.3 Decarboxylation of the malonate products provides arylacetic acid derivatives, which are synthetically useful intermediates. Alternatively, alkylation followed by decarboxylation provides α-arylcarboxylic acid derivatives that are also highly valued synthons. Arylations of diethyl malonate with chloroarene electrophiles proceed smoothly in the presence of Pd(dba)2 and Q-Phos, while reactions of chloro- or bromoarenes with ethyl cyanoacetate require Pd(dba)2 or [Pd(allyl)Cl]2 along with Q-Phos depending on the identity of the substrates.
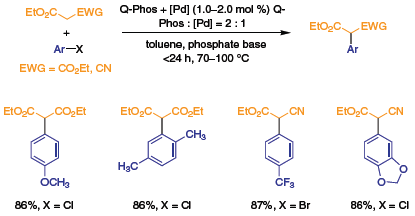
Scheme 1.
More recently, the Hartwig group demonstrated the first general method for the α-arylation of amides, via their zinc enolates.4 While zinc amide enolates are not common intermediates, they are easily generated by reaction of an alkali enolate with ZnCl2. Alternatively, they can also be generated via the reaction of activated Rieke® zinc with the necessary α-bromoamide, although this approach is limited to those bromoamides which are readily available. The use of zinc amide enolates over alkali metal enolates is preferred because the former exhibit greater functional group tolerance in coupling reactions.
As shown in Scheme 2, treatment of dialkyl acetamides or dialkyl propionamides with s-BuLi, followed by transmetalation with ZnCl2, generates an intermediate organozinc halide, which readily couples with aryl bromides in the presence of Q-Phos and Pd(dba)2 to give the α-arylamide. Electron-rich or electron-poor bromoarenes, vinyl bromides, and heteroaryl bromides are all reactive in the C–C bond-forming reaction. Finally, morpholine amides, which can behave similarly to Weinreb amides in their functional group manipulations,5 also undergo arylation.
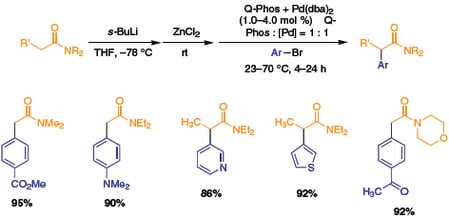
Scheme 2.
Materials
References
To continue reading please sign in or create an account.
Don't Have An Account?