Analysis of PFAS Extractables in Filtration Products Using Modified EPA 537.1
Section Overview
- Contaminants in PFAS Testing
- Testing Millex® PES, Nylon, and MCE Syringe Filters With Water Samples Using Modified EPA 537.1
- Testing Millipore® Polypropylene, PES, and MCE Cut Disc Filter Membranes With Water Samples Using Modified EPA 537.1
- Filter Considerations for PFAS Sample Preparation
- Recommended Filtration Products
Contaminants in PFAS testing
A key consideration for any PFAS method is to avoid contamination that can impact the accuracy of data, including those coming from sample preparation techniques such as filtration. Currently, most PFAS analytical methods are for “clean” matrices, such as drinking water, and often do not require filtration as a part of sample preparation. However, methods such as SW-846 Method 8327, ASTM D7968, ASTM D7979, EPA 1633, ASTM D8421, and ISO 21675 involve matrices that could have a higher degree of particulates, such as wastewater. Particulates in solution must be removed prior to LC-MS/MS, as they can be detrimental to sample analysis, column longevity, resulting data quality, and overall instrument function. These methods identify the need for filtration using membranes in a syringe filter format.
A modified EPA 537.1 method was used to demonstrate that Millex® polyethersulfone (PES), nylon, and mixed cellulose esters (MCE) syringe filters as well as Millipore® polypropylene, PES, and MCE cut disc filter membranes do not exhibit detectable levels of PFAS contaminants resulting from fluorinated extractables.
Testing Millex® PES, MCE, and Nylon Syringe Filters with Water Samples using Modified EPA 537.1
Materials and Methods
Three lots of PES, three lots of nylon, two lots of nylon-HPF (nylon membrane with a glass fiber prefilter for high-particulate filtration), and single lots of MCE Millex® syringe filter devices were tested for PFAS extractables according to EPA Method 537.1. Some PFAS compounds not required by the method, including next-generation PFAS compounds and fluorotelomer sulfonates, were also included. EPA 537.1 does not require sample filtration, but filtration was used to provide a clean sample to test extractable contamination levels from the syringe filter.
An overview of the modified method EPA 537.1 using internal standards is described in the workflow below, with the LC-MS/MS conditions in Table 1. Testing was conducted in collaboration with SGS North America at their Orlando, FL facility. Briefly, a 250 mL PFAS-free DI water sample was spiked with surrogates. The internal standard spike of 0.08 ppb was used for QC blanks. To determine if sample filtration media contributes to PFAS contamination, the entire sample was passed through the filter and into a styrene divinylbenzene (SDVB) SPE cartridge. The sample bottles and tubes were rinsed with basic methanol and also passed through the filter and into the cartridge. The entire sample was then subjected to SPE and concentrated to 1 mL in 96:4% (v/v) methanol:water prior to LC-MS/MS analysis using a C18 column. Analysis was performed using internal standards. C13-labeled standards were used in this study. The filters tested included:
- Three lots of Millex®-GP syringe filters (non-sterile, 33 mm filter with Millipore Express® PLUS PES membrane) in both 0.22 µm (Cat. No. SLGP033N) and 0.45 µm (Cat. No. SLHP033N) pore sizes
- Three lots of Millex® nylon syringe filters (non-sterile, 33 mm filter with nylon membrane) in 0.20 µm pore size (Cat. No. SLGN033N),
- Two lots of Millex® nylon-HPF syringe filters (non-sterile, 25 mm filter with nylon membrane and glass fiber prefilter) in 0.20 µm pore size (Cat. No. SLGNM25)
- Single lots of Millex® MCE syringe filters (non-sterile, 25 mm filter with MCE membrane) in 0.2 µm (Cat. No. SLGS025NB), 0.45 µm (Cat. No. SLHA025NB), and 0.8 µm (Cat. No. SLAA025NB) pore sizes, tested in triplicate
Workflow for method used for modified EPA 537.1:
Workflow
Filter
Samples are filtered using a Millex® syringe filter.
Solid Phase Extraction
Analytes are extracted from the sample by solid phase extraction (SPE).
Concentration
Extracts are concentrated by evaporation with nitrogen using a water bath.
LC-MS/MS
Concentrated extracts are analyzed by LC-MS/MS.
Results
There were no detectable PFAS contaminants in any of the Millex® devices tested using modified EPA 537.1 above the reporting limits (RL) or the minimum detection limits (MDL), even given the very low thresholds of 0.0020-0.0080 ppb and 0.0010-0.0020 ppb, respectively, depending on the compound (Table 2). The results were the same for the three different lots of the 0.22 and 0.45 µm PES membranes, three different lots of 0.20 µm nylon membranes, two different lots of 0.20 µm nylon-HPF membranes, and 0.2, 0.45, and 0.8 µm MCE membranes tested in triplicate. These results suggest that PES, nylon, nylon-HPF, and MCE Millex® filtration devices are reliable and appropriate to utilize in the filtration of water samples in preparation for the analysis of these PFAS compounds.
aThree replicate devices of each of three lots tested, no PFAS compound detected in any lot above RL or MDL
bThree replicate devices of each of two lots tested, no PFAS compound detected in any lot above RL or MDL
cThree replicate devices of one lot tested, no PFAS compound detected in any lot above RL or MDL
Abbreviations: RL = reporting limit; MDL = minimum detection limit; PES = polyethersulfone; HPF = high particulate filter; MCE = mixed cellulose esters; ND = not detected
Table 2. Detection of PFAS contaminants after filtration with Millex® PES, nylon, and MCE devices using modified EPA 537.1.
Recovery
In this study, sample tubes and sample bottles were rinsed with basic methanol. The recoveries of the C-13-labeled standards were within the acceptable QC range for the method. However, recovery varied from filter material to filter material (Figure 1) and from compound to compound. For example, nylon-based filter devices demonstrated lower recoveries than those with PES. In the case of nylon membranes, non-specific adsorption of internally spiked standards and sample can be reduced using a methanol rinse. Similar results were found using PES membranes alone (without housing and filtered using a 25 mm Swinnex® filter holder device).
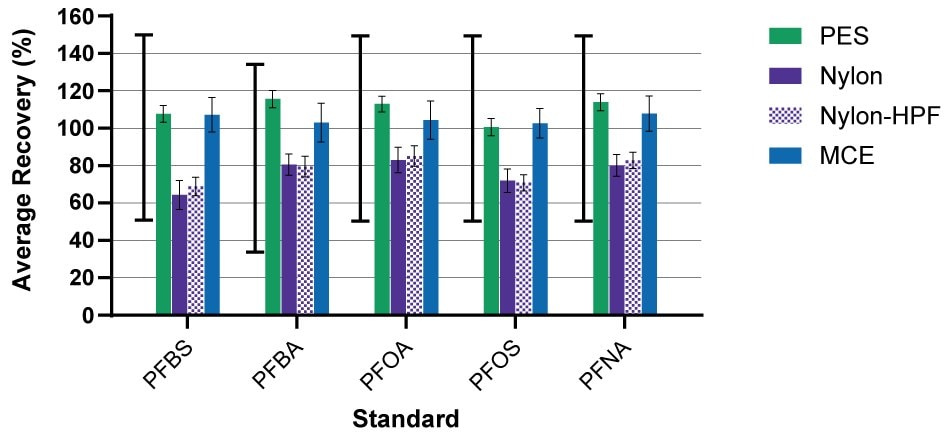
Figure 1.The average percent recovery of C13-labeled standards for PFBS, PFBA, PFOA, PFOS and PFNA after filtration with 0.22 µm PES (mean ± standard deviation (STDEV), n=9 replicates over 3 lots), 0.2 µm nylon (mean ± standard deviation (STDEV), n=9 replicates over 3 lots), 0.2 µm nylon-HPF (mean ± STDEV, n=6 replicates over 2 lots), and 0.22 µm MCE (mean ± STDEV, n=3 replicates over 1 lot) Millex® syringe filter devices. The acceptable QC range for recovery of internal standards is demonstrated by the solid black, capped line to the left for each compound.
Testing Millipore® Polypropylene, PES, and MCE Cut Disc Filter Membranes with Water Samples using Modified EPA 537.1
Syringe filter devices are the most recommended and preferred format for filtering samples for LC-MS/MS analysis of PFAS due to ease of use and the range of small volumes that can be processed (10-100 mL). There are instances, however, when syringe filters may not be the best option for filtration; for example, when there are no commercially available syringe filters suitable for a specific application. In these instances, an alternative must be considered. A syringe filter-like device, such as a Swinnex® holder, is a viable alternative. This pressure-driven device holds any cut disc filter membrane of a specific size (13 mm or 25 mm diameter) and is operated in the same way as a traditional syringe filter, thus converting any membrane material into a syringe filter format.
Polypropylene is a commonly preferred material for sample preparation and filtration. Polypropylene is a durable material compatible with a broad range of solvents and temperatures and demonstrates low extractables, making it appropriate specifically for PFAS-related sample and mobile phase preparation. A challenge with polypropylene is that it is naturally hydrophobic, which makes it challenging to filter aqueous samples. Most commercially available polypropylene disc filters are hydrophobic, such as Millipore® polypropylene cut disc filter membranes (Cat. No. PPTG04700 and Cat. No. PPTH04700). Though appropriate for solvents such as methanol, it can be challenging to filter aqueous samples. In a few cases, polypropylene can be found in a hydrophilic format (Millipore® hydrophilic polypropylene cut disc membrane filters, Cat. No. PPHG04700 and Cat. No. PPHH04700). These filters are suited to handle aqueous samples. Thus, realizing the potential for polypropylene material to be used within the context of a variety of PFAS workflows, including mobile phase filtration, we determined the level of PFAS extractables that these cut filter membranes release.
Materials and Methods
Swinnex® Filter Holder Assembly
Hydrophilic and hydrophobic Millipore® polypropylene (PP) cut disc filter membranes of 0.2 µm and 0.45 µm pore sizes, Millipore® PES cut disc filter membranes of 0.22 µm and 0.45 µm pore sizes, and Millipore® MCE cut disc filter membranes of 0.45 µm, 0.8 µm, and 5.0 µm pore sizes were tested for PFAS extractable content. Swinnex® devices (25 mm diameter) were used to convert the various disc membrane filters into a Luer-lock based syringe filter device, according to Figure 2. Once assembled, the Swinnex® device can be connected to a Luer-lock syringe barrel with the material to be filtered. Filtration was then performed as with other syringe filter devices. For every disc replicate, a new and clean Swinnex® device was used.

Figure 2A.O-ring placement
Figure 2B.Filter handling

Figure 2C.Filter placement

Figure 2D.Gasket fastening
Figure 2E.Assembled Swinnex® filter holder
Modified EPA 537.1
In this part of the study, nine types of 25 mm cut disc filter membranes were tested:
- 0.2 µm hydrophobic PP (Cat. No. PPTG02500)
- 0.45 µm hydrophobic PP (Cat. No. PPTH02500)
- 0.2 µm hydrophilic PP (Cat. No. PPHG02500)
- 0.45 µm hydrophilic PP (Cat. No. PPHH02500)
- 0.22 µm PES (Cat. No. GPWP02500)
- 0.45 µm PES (Cat. No. HPWP02500)
- 0.45 µm MCE (Cat. No. HAWP02500)
- 0.8 µm MCE (Cat. No. AAWP02500)
- 5.0 µm MCE (Cat. No. SMWP02500)
Once each cut disc filter membrane was placed securely into a Swinnex® device, 250 mL water samples spiked with surrogates were passed through each filter and into a styrene divinylbenzene (SDVB) SPE cartridge using EPA 537.1 for drinking water matrices as a guideline. The entire sample was subjected to SPE and concentration, according to procedures outlined in the workflow above. LC-MS/MS with a C18 column was performed according to conditions in Table 1 and analysis was carried out using internal standards to determine if there were levels of extractables present in each cut disc filter membrane. One lot (n=3 filters per lot) was tested per membrane type.
It is important to note that it is difficult to flow pure water through hydrophobic PP cut disc filter membranes; thus, for improved water flow for these samples, cut disc filter membranes were pre-wetted in methanol prior to filtering 250 mL water. There was no need to pre-wet hydrophilic PP cut disc filter membranes.
Results
As was found for Millex® syringe filter devices, there were no detectable PFAS contaminants in any of the polypropylene, PES, or MCE cut disc filter membranes according to modified EPA 537.1 above the RL, ranging from 0.0020-0.0080 ppb, or the MDL, ranging from 0.0010-0.0020 ppb (Table 3). This indicates that these membranes do not have PFAS extractables at these limits and could be used for PFAS applications where filtration is needed for sample preparation.
However, for only the hydrophobic polypropylene cut disc filter membranes, there were four compounds (perfluoro-n-dodecanoic acid (PFDoDA), perfluoro-n-tridecanoic acid (PFTrDA), perfluoro-n-tetradecanoic acid (PFTeDA), and N-ethyl perfluorooctanesulfonamidoacetic acid (N-EtFOSAA)) where there were no detectable PFAS compounds, but the associated standards of 13C2-PFDoA, 13C2-PFTeDA and D5-NEtFOSAA, demonstrated recoveries outside of the control limits of 40-140%, 30-130%, and 40-140%, respectively. These compounds demonstrated average recoveries of approximately ~15-25% (for PFDoDA, PFTrDA, and PFTeDA) and 30-33% (for N-EtFOSAA). This indicates that non-specific adsorption of these compounds onto hydrophobic polypropylene could be significant in water. Considering the long chain lengths and bulky functional groups of these compounds, there is a potential for hydrophobic and steric interactions with filtration media and other consumables. Interestingly, hydrophilic polypropylene recoveries remained within control limit ranges for all compounds, indicating that hydrophilization of the membrane material reduced any non-specific interactions with PFAS standards.
aThree replicate devices per lot tested, three lots per catalog number tested
bThree replicate devices per lot tested, two lots per catalog number tested
cThree replicate devices per lot tested, one lot per catalog number tested
dAssociated ID standard outside of control limits for all three devices tested; 13C2-PFDoDA, 13C2-PFTeDA, d5-EtFOSAA
Abbreviations: RL = reporting limit; MDL = minimum detection limit; PP = polypropylene; philic = hydrophilic; phobic = hydrophobic; PES = polyethersulfone; MCE = mixed cellulose esters; ND = not detected
Table 3. Detection of PFAS contaminants in water after filtration with hydrophobic 0.2 µm and 0.45 µm polypropylene, hydrophilic 0.2 µm and 0.45 µm polypropylene, 0.22 µm and 0.45 µm PES, and 0.45 µm, 0.8 µm, and 5.0 µm MCE cut disc filter membranes in Swinnex® devices using modified EPA 537.1.
Recovery
Hydrophobic Versus Hydrophilic Polypropylene Membranes
In water samples, the hydrophilic polypropylene cut disc filter membranes tested demonstrated recovery of internal standards within the QC range, while the hydrophobic cut disc filter membranes did not (Figure 3). The recoveries for 13C2-PFDoDA, 13C2-PFTeDA and D5-EtFOSAA consistently demonstrated recovery outside of the QC range for hydrophobic polypropylene, making it difficult to quantify the true PFAS content for these compounds. Given that PFDoDA, PFTeDA, and PFTrDA are long-chain PFCA molecules, observed loss may be due to steric hindrance and hydrophobic interactions with the membrane material or Swinnex® housing material.

Figure 3. The average percent recovery of C-13 labeled standards for hydrophilic versus hydrophobic polypropylene cut disc membrane filters in water, for the perfluoroalkyl carboxylic acid class of PFAS only. Values are mean ± standard deviation, n=3 replicates. The QC range for the standards varies by compound, from left to right: 35-135% (13C4-PFBA), 50-150% (13C5-PFPeA through 13C6-PFDA), 40-140% (13C2-PFDoDA), 30-130% (13C2-PFTeDA).
PES and MCE Membranes
The recoveries of all 13C-labeled standards were within the acceptable QC range for the method when using both MCE and PES 0.45 µm cut disc filter membranes. However, recovery varied slightly based on both filter material and compound (Figure 4). For example, 0.45 µm PES filters in both syringe filter and cut disc filter membrane format demonstrated generally higher recoveries of nearly all tested PFAS compounds compared to MCE. The largest difference in recovery for any condition was with PFOSA, where PES showed between 108-120% recovery versus MCE which was considerably lower (still within the QC range), between 36-60% recovery.

Figure 4.The average percent recovery of 13C-labeled PFAS standards after filtration with 0.45 µm MCE and PES Millipore® cut disc filter membranes (mean ± standard deviation, n=3 replicates).
Filter Considerations for PFAS Sample Preparation
There are many considerations when selecting a filter for a particular analytical method. Filters should be compatible with sample and chemical components, exhibit low levels of extractables that might interfere with data interpretation, and yield high recovery for the analyte-of-interest. For PFAS methods, it is necessary to carefully consider filter material, pore size, format, manufacturer, and method parameters for the best filter performance. These data show that Millex® PES, nylon, nylon-HPF, and MCE syringe filters as well as Millipore® hydrophilic polypropylene, hydrophobic polypropylene, PES, and MCE cut disc filter membranes maintain high PFAS recoveries and do not demonstrate levels of PFAS contamination, and may therefore be suitable for use in PFAS test methods.
Recommended Syringe Filters
Recommended Cut Disc Membrane Filters
Recommended Filter Holders
To continue reading please sign in or create an account.
Don't Have An Account?