ChIC/CUT&RUN Kits
- Protein-DNA interactions and the Chromatin Immunoprecipitation (ChIP) method
- Overview of ChIC and CUT&RUN using a modified micrococcal DNA nuclease (MNase)
- ChIC/CUT&RUN kits introduction
- Application data for ChIC/CUT&RUN kits
- ChIC and CUT&RUN data analysis tool
Protein-DNA interactions and the Chromatin Immunoprecipitation (ChIP) method
Protein-DNA interactions are essential to many biological processes, such as DNA replication and repair, transcription and regulation of gene expression. Understanding the specificity with which proteins bind to DNA sequences in varying physiological conditions is of considerable importance. Among many techniques interrogating protein-DNA interactions, chromatin immunoprecipitation (ChIP) has been widely used to interrogate protein-DNA interactions. When researchers utilize ChIP, cells are cross-linked with formaldehyde, chromatin is fragmented and solubilized, and immunoprecipitation is applied to solubilized chromatin. Although the readout methods for ChIP have evolved over the years from endpoint PCR to high-throughput sequencing, the fundamentals of ChIP have remained unchanged. However, issues remain for this technique, including high background that limits sensitivity, challenges wit high amounts of cellular input, and artifacts resulting from cross-linking and protein solubilization.
Overview of ChIC and CUT&RUN using a modified micrococcal DNA nuclease (MNase)
Facing the challenges of utilizing the ChIP technique when dealing with insoluble proteins, researchers developed an elegant alternative strategy termed chromatin immunocleavage (ChIC)1. ChIC detects the binding sites of transcription factors in the genome by targeting a modified micrococcal nuclease (MNase), conjugated with protein A (pA-MN), using a specific antibody. The modified MNase specifically cleaves DNA at regions interacting with a protein of interest only when Ca2+ ions are present, therefore allowing for controlled DNA cleavage at the antibody binding site. This approach allows for mapping proteins with a 100–200 bp resolution and excellent specificity, compared to conventional ChIP methods 1.
Adapting from ChIC, researchers further optimized the protocol and combined ChIC with deep sequencing to detect genome-wide transcription factor binding sites and histone modifications on native chromatin with <5 bp resolution. This method combining ChIC with deep sequencing is termed Cleavage Under Targets and Release Using Nuclease (CUT&RUN)2.
With the ChIC and CUT&RUN methods, only the targeted fragments are released into solution while the majority of DNA is left behind, leading to an exceptionally low background level (Figure 1).
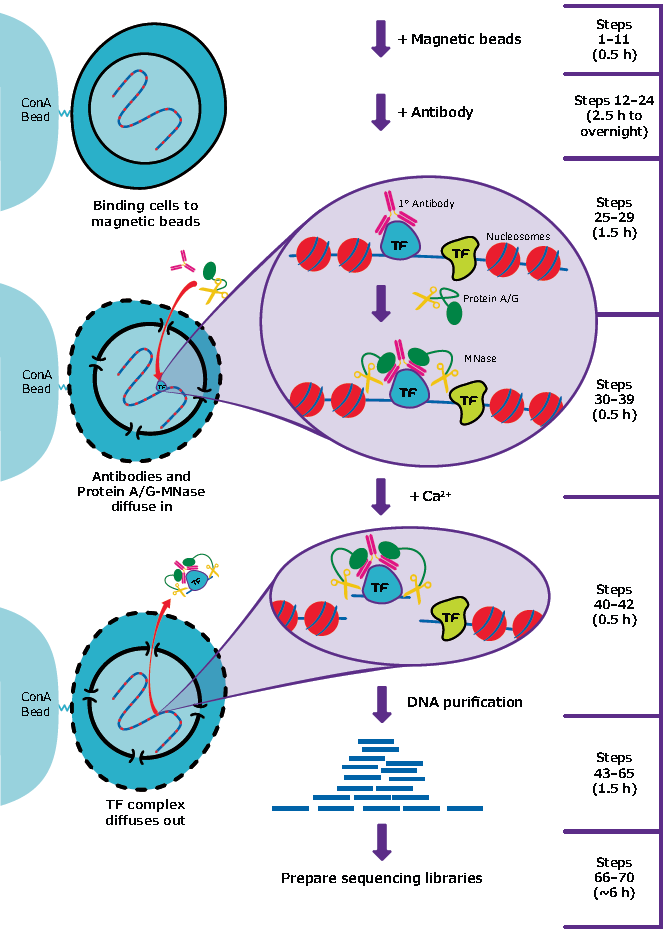
Figure 1: Overview of ChIC and CUT&RUN method.To perform ChIC and CUT&RUN, cells are harvested and bound to concanavalin A–coated magnetic beads. Cell membranes are permeabilized with digitonin to allow the specific antibody to find its target. Following antibody incubation, beads are briefly washed and incubated with pA-MN. Cells are chilled to 0 °C, and digestion begins with addition of incubation buffer containing Ca2+. Reactions are stopped by chelation and the DNA fragments released into solution by cleavage are extracted from the supernatant.
As a result, ChIC and CUT&RUN delivers high-quality data using only 100 cells for a histone modification analysis and 1,000 cells for a transcription factor binding site analysis. In contrast to ChIP, ChIC and CUT&RUN is free of solubility issues and DNA accessibility artifacts from cross-linking. ChIC and CUT&RUN outperforms ChIP in terms of resolution, signal-to-noise ratio, and depth of sequencing required. Below is a summary table of advantages of ChIC and CUT&RUN compared with X-ChIP-seq (Table 1).
ChIC/CUT&RUN kits introduction
We developed three ChIC/CUT&RUN kits (Cat. #: CHR100-102)” based on ChIC and CUT&RUN techniques, with an addition of pG-MN or pA-MN/pG-MN protein cocktail to provide more options for antibody choice. pA-MN has a higher affinity to most antibodies, including rabbit IgG, whereas pG-MN is more suitable for antibodies that have low affinity to protein A, for example mouse IgG1.
These kits provide the following benefits for your genome-wide profiling of chromatin protein interactions:
- Extremely low signal-to-noise ratio compared to cross-linking ChIP-seq
- Removal of cross-linking, sonication, and high-speed spin steps
- Reduction in the required number of starting cells (minimum 100 cell for histone modification and 1,000 cells for a transcription factor)
- Significantly reduced procedure time – from cells to purified DNA all within one day (minimum 3 hours)
- No special equipment needed
The ChIC/CUT&RUN kits can be used for many basic can be used for many basic and translational applications for which only limited numbers of cells are available (standard cross-linking ChIP protocols not suitable for this application), such as developmental biology studies, and the analysis of clinical samples or cells obtained after fluorescence-activated cell sorting or dissection. By eliminating sonication and high-speed spin steps to remove insoluble materials and attaching cells to magnetic beads, these ChIC/CUT&RUN kits can be potentially used for high-throughput applications as well.
Application data for ChIC/CUT&RUN kits
Below are sequencing results after performing ChIC and CUT&RUN assay using the ChIC/CUT&RUN kits (Figure 2-3).
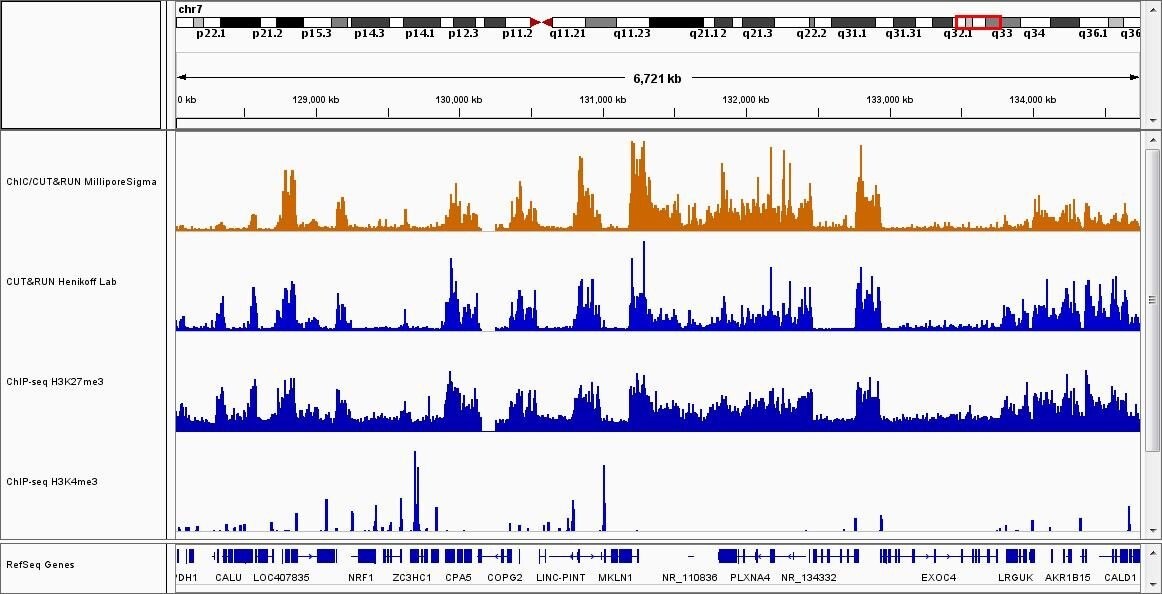
Figure 2: Sequence results for ChIC and CUT&RUN assay (H3K27me3).ChIC and CUT&RUN assay was performed with anti-H3K27me3 antibody and 2x105 cells (1st track). The results were compared with published CUT&RUN assay results (2nd tack)and ENCODE ChIP-seq data (3rd track) 2. Anti-H3K4me3 ChIP-seq results from ENCODE are also shown as a negative control (4th track).
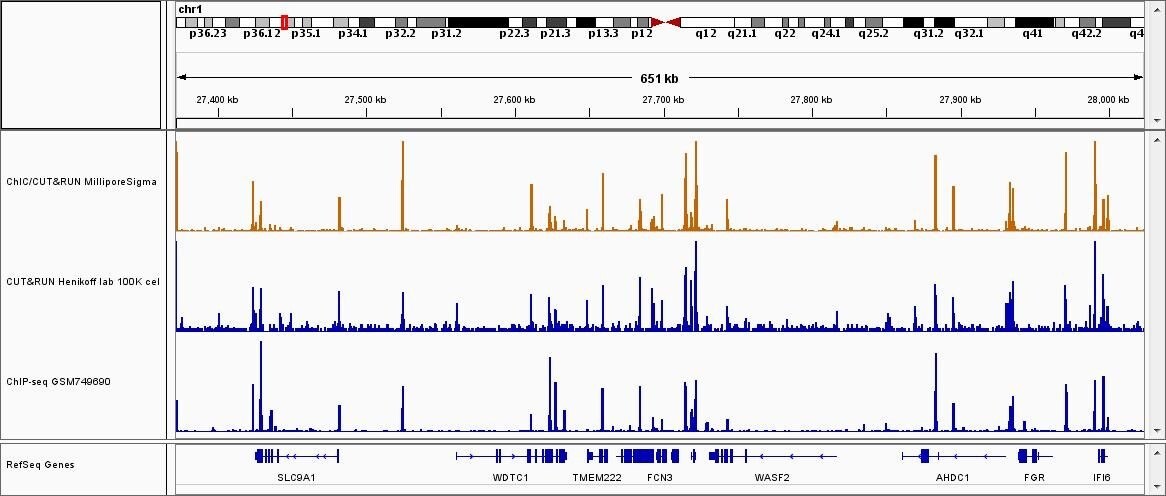
Figure 3.Sequence results of ChIC/CUT&RUN assay (CTCF). ChIC and CUT&RUN assay was performed with anti-CTCF antibody (Cat. #: 07-729) and 2x105 of K562 cells (1st track). These results were compared with published CUT&RUN assay results (GSE104550) (2nd tack) and ENCODE ChIP-seq data (GSM749690) with the same antibody (3rd track) 2.
ChIC and CUT&RUN Data Analysis Tool
We understand that even after running a successful ChIC and CUT&RUN experiment, you may still face challenges in processing and analyzing the resulting data.Therefore, we created a ChIC and CUT&RUN data analysis tool to enable you to perform the data analysis yourself. This tool was developed based on commonly used and validated algorithms, such as MACS2 for peak calling, with consideration of absence or presence of replicates and barcodes. The tool is very easy to use, does not require prior experience with bioinformatics, and is free to ChIC/CUT&RUN kit customers.
For more information, view the ChIC and CUT&RUN Data Analysis Tool Overview and the ChIC and CUT&RUN Data Analysis Tool FAQ. Your order ID can be found on the packing slip, the order confirmation or retrieved by calling your local Customer Service.
References
To continue reading please sign in or create an account.
Don't Have An Account?