Nanostructured Catalyst for Direct Alcohol Low-Temperature Fuel Cells
Introduction
At a time when the world is committed to changing its energy matrix, fuel cells are back on the scene as a promising source of energy. Fuel cells can convert chemical energy into electrical energy efficiently. Conceptually, fuel cells are very similar to the Daniell cell, which is often taught in introductory chemistry courses. In a Daniell cell, the anode (usually a metal like zinc) is oxidized and the oxidized metal ions dissolve into the electrolytic solution while metal ions at the cathode surface are reduced. Similarly, in a fuel cell, molecules (for example, H2 and O2) are inserted into the device and are oxidized and reduced at the electrodes.1
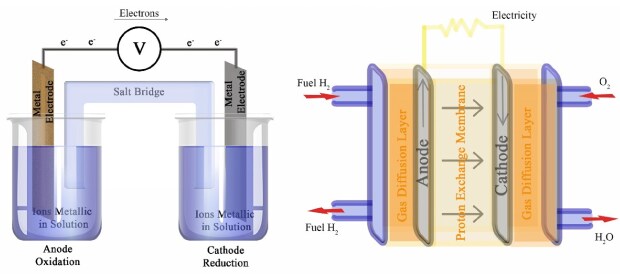
Figure 1.Diagram of the Daniell Cell and the Hydrogen Fuel Cell devices.
Conceptually, the hydrogen fuel cell is one of the cleanest energy sources, consuming only hydrogen and oxygen and releasing only water; however, hydrogen fuel cells face technological challenges. For example, producing, storing, and transporting hydrogen is still expensive and incurs considerable risks, even though the technology to accomplish these processes has greatly evolved. In addition, the loss of gas during storage and transport remains a challenge.2
One strategy to overcome this problem was the adoption of hydrogen-generating devices, such as thermo-reformers, to generate hydrogen on demand for use in the fuel cell.3–4 The downside to this strategy is that the power generation system occupies more volume due to this extra device.
Direct Methanol Fuel Cells
Direct alcohol oxidation cells started with the simplest alcohol, methanol, which is a liquid that is easy and cheap to store and transport, and has a high energy density of 6.09 kWh kg-1, including an existing distribution network due to being an industrial input.5 Initial studies identified platinum (Pt) as a promising catalyst for the methanol oxidation reaction (MOR), but two main obstacles quickly emerged: 1) Pt is expensive and 2) Pt in MOR is prone to poisoning by carbon monoxide.
Fortuitously, the beginning of studies on the methanol oxidation reaction (MOR) happened concomitantly with the development of new methods for preparing nanostructured materials. Researchers found that nanostructured materials as catalysts for MOR may offer a solution to both of the obstacles faced by traditional Pt catalysts.
The findings on nanostructure platinum galvanized interest in studying direct methanol fuel cells (DMFC). One strategy that emerged to address the catalytic poisoning was to use reactive oxygen species (ROS), made by the water activation product, to constantly remove some of this catalytic poison. A second strategy, adopted from research on the electrolysis reaction, soon followed, to add other metals such as ruthenium (Ru) together with Pt that would act in a bifunctional mechanism (Figure 2). In this mechanism, the ROS reacts with the strongly adsorbed CO on the electrode surface oxidizing it to CO2.6
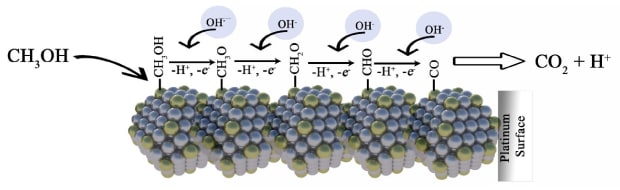
Figure 2.Bifunctional mechanism of the methanol oxidation on the platinum surface catalyst.
During this period, not only the addition of a second, sometimes a third, and even a fourth metal was studied, but also the optimal composition, which, in addition to increasing the activity of the catalyst, also reduced the use of platinum in the catalyst. One particularly successful catalyst developed in this period is PtRu in the composition of 50% in atoms among the metals, which to this day is still considered the benchmark for MOR, even though superior materials have already been presented in the literature.6
Researchers developed another way to obtain ROS for CO removal, by adding rare earth oxides to the catalyst. These rare earth oxides, such as ceria, act as an oxygen buffer near the surface of the material. By changing its oxidation state easily according to the presence or absence of oxygen near its interface, ceria creates a chemical environment on the electrode surface that favors CO oxidation.7
Yet another approach is to tailor the Pt catalyst. For example, changing the platinum D-band density decreases the CO adsorption energy and limits catalytic poisoning. This strategy is based on the insertion of a metallic heteroatom in the crystalline structure of platinum, obtaining alloys, the named electronic effect.8 The CO oxidation at low overpotentials can also be achieved by selecting preferred platinum faces, for example, low-index crystalline planes, which have adequate surface energy to carry out the oxidation of CO and water at low overpotentials. Similarly, the same effect can be obtained by creating surface defects as reported by Ramos et al.9
Direct Ethanol Fuel Cell (DEFCs)
Studies with alcohols have expanded to the oxidation of ethanol, as a substitute for methanol, because it is less toxic, has an energy density greater than that of methanol (8.02 kWh kg-1), and mainly to be a renewable fuel obtained from biomass. The use of alcohol with a slightly longer chain brought with it the problems of methanol and a new challenge, the breaking of the C-C bond. Pt-based electrocatalysts were not efficient for this purpose; however, the alloy PtSn 3:1 was appointed as the benchmark due to the high powers obtained.10
Even the PtSn alloy does not show the complete oxidation of alcohol to CO2, and in search of these scientists began a new search for solutions that could help to overcome this challenge. One of the options found was the addition of Rh to the catalyst because this metal has an electron affinity for the C-C bond.11 However, studies that explored this option showed only an increase in CO2 generation, but this was reflected in smaller currents. This fact combined with the high costs of Rh reduced the use of this noble metal as the catalyst for the ethanol oxidation reaction.
Another strategy adopted was the use of catalysts with steps and terraces to break the C-C bond. However, these stepped and terraced materials tend to restructure to more stable forms, which makes it difficult to maintain the effect in the long term. In addition, stepped and terraced materials are difficult to synthesize, which discourages the application of this kind of material on a large scale.
One of the additional challenges that ethanol brings is that the partial oxidation of ethanol yields stable products like acetaldehyde and acetic acid. On the one hand, the incomplete oxidation of ethanol reduces the energy density that can be utilized, which is a drawback, but on the other hand, these products do not strongly adsorb to the catalyst and do not function as catalytic poisons. The most active catalysts for the ethanol oxidation reaction normally affect the kinetics of acetaldehyde or acetic acid formation, which can go through several oxidation pathways12 as shown in Figure 3. To date, catalyzing the partial oxidation of ethanol is much better understood than the slow step of breaking the C-C bond.
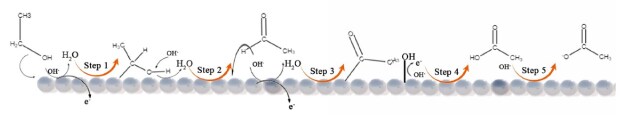
Figure 3.Schematic representation of the pathways of ethanol oxidation on the metallic catalytic surface.
Direct Glycerol Fuel Cell
With the popularization of biodiesel, the production of glycerol grew beyond what the market needed, transforming this alcohol from a commodity to an environmental problem. Being another fuel from biomass, this alcohol with its three hydroxyls has the breaking of the C-C bond facilitated by the steric effect of the oxidation of any of the three functional groups, but, like methanol, it has similar problems with CO.
Usually, the potency densities obtained by the oxidation of glycerol are lower than those of the other alcohols already presented, but the diversity of products formed during the oxidation of glycerol opened the eyes of the scientific community to the possibilities of co-generation of energy and chemicals.13 Figure 4 shows the reaction pathways of glycerol oxidation.14
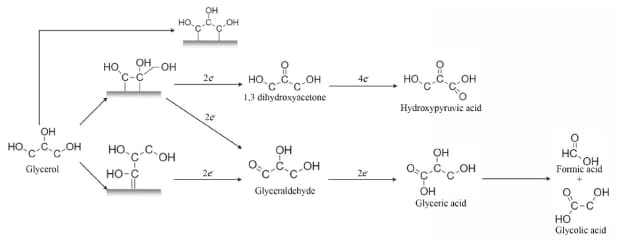
Figure 4.Reaction mechanism of glycerol oxidation in an acidic environment to produce different products.
Anion Exchange Membrane Fuel Cells
Around 2010 the development of anion exchange membranes brought the possibility of carrying out the oxidation of alcohols in an alkaline medium and with that some advantages, such as greater ease in the oxidation of alcohols whose hydroxyls are more easily deprotonated, the alkaline medium also reduces the possibility of intermediates to be formed during oxidation. Since aldehydes are unstable at high pH and most importantly, many metals that only activate water at neutral pHs become active at high pH in the oxidation of alcohols. One such metal is Pd, which is around 30 times more abundant than platinum. Other transition metals also appeared as viable, such as Ni, Cu, Au, Ag.15
Studies on the oxidation of alcohols in the alkaline medium have gone into great depth in the last few decades, but anion exchange membranes have not evolved at the same rate as catalysis, and are still not very durable.16 The alkaline medium suffers from another type of catalytic poisoning, the coverage of the site by the deposition of solid carbonate deposited on the catalytic layer, this carbonate from the precipitation of CO and CO2 in an alkaline medium prevents the arrival of new alcohol molecules to the catalytic surface.
Opportunities and Outlook
Nanostructured materials made it possible to study low-temperature fuel cells, especially those fueled with alcohol, which brings the possibility of obtaining renewable energy from alcohols such as ethanol and glycerol. Anion exchange membranes brought the possibility of decreasing the dependence of Pt on the catalyst, but they need to evolve to increase durability.
Regardless of cell type, for ethanol and glycerol, the most active catalysts are not those that promote complete oxidation of alcohol, which implies a lower use of energy density, however, makes it possible to obtain chemically stable partially oxidized products. This allows the cells to do something beyond their initial purpose of generating energy, but rather the co-generation of energy and chemicals.
Related Materials
References
To continue reading please sign in or create an account.
Don't Have An Account?