RESOMER® Biodegradable Polymers for Medical Device Applications Research
RESOMER® Polymers by Evonik Corporation
Introduction
Polymers with controlled biomedical degradation characteristics can be used as an important part of tissue engineering and drug delivery therapies. Many types of natural and synthetic biodegradable polymers have been investigated for medical and pharmaceutical applications. While use of natural polymers, such as cellulose and starches, is still common in biomedical research, synthetic biodegradable polymers are increasingly used in pharmaceutical and tissue-engineering products. Synthetic polymers can be prepared with chemical structures tailored to optimize physical properties of the biomedical materials and with well-defined purities and compositions superior to those attainable when using natural polymers. There are now several established chemical classes of synthetic biodegradable polymers that offer good biocompatibility and can be selected to tune rates of biodegradation and mechanical strength.1
Poly(glycolic acid) (PGA), Poly(lactic acid) (PLA) and their copolymers have been researched for a wider range of applications than any other type of biodegradable polymers. PLA/PGA are biodegradable polyesters that degrade in the body by simple hydrolysis of the ester backbone to non-harmful and non-toxic compounds. The degradation products are either excreted by the kidneys or eliminated as carbon dioxide and water through well-known biochemical pathways. Current applications of the polymers include surgical sutures and resorbable implants, with significant interest to further expand the use of these materials to drug encapsulation and delivery applications. Since PLA/PGA polymers are considered safe, non-toxic and biocompatible by regulatory agencies in virtually all developed countries, additional applications of these materials can be brought to market sooner and are more cost effective than those utilizing novel polymers with unproven biocompatibility.
We are pleased to offer a comprehensive selection of PLA/PGA RESOMER® polymers manufactured by Evonik Röhm Pharma GmbH. Below you will find basic information about properties of the RESOMER® polymers and a product table listing available RESOMER® products. RESOMER® polymers from our catalog are sold for research and development applications only. Chemically identical GMP materials are available directly from Evonik Röhm GmbH.
Properties of PLA/PGA Polymers
PGA, PLA and their copolymers are some of the most frequently used biodegradable polymer materials in part because their properties that can be tuned by changing the polymer composition within the basic PLA/PGA theme. The following classes of RESOMER polymers are available in our catalog:
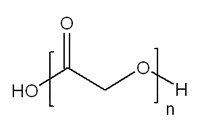
Figure 1. General Structure of PGA
Poly(glycolic acid) (PGA)
PGA (457620) is a highly crystalline material, which boasts a high melting point (225-230 °C) and variable solubility in organic solvents that is generally low and depends on polymer molecular weight. It is still susceptible to hydrolysis due to the ester bond in the polymer backbone. In spite of its low solubility, this polymer has been fabricated into a variety of forms and structures. Techniques used to develop PGA-based structures include extrusion, injection and compression molding as well as particulate leaching and solvent casting. Fibers of PGA have a high strength and modulus (7 GPa) and are particularly rigid,2 which has promoted investigation to their use in bone internal fixation devices. However, lower solubility and brittle nature of PGA materials can limit their utility in some applications.
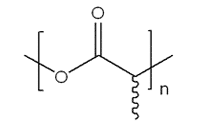
Figure 2. General Structure of PLA
Poly(lactic acid) (PLA)
Unlike glycolide, lactide is a chiral molecule and exists in two distinct optically active forms – L-lactide and D-lactide. When each of these monomers is polymerized, the resulting polymer is semi-crystalline. Polymerization of a racemic mixture of L- and D-lactides forms poly-D,L-lactide (PDLLA), which is amorphous and has a glass transition temperature of 55-60 °C. The degree of crystallinity can be tuned by altering the ratio of D to L enantiomers within the polymer. Selection of the PLA stereochemistry can have a major effect on the polymer properties, processability and biodegradability. Poly (L-lactide) or PLLA is often the polymer of choice for cast/extruded biomedical devices because it breaks down into L(+)-lactic acid units, which is the naturally occurring stereoisomer and is therefore excreted with minimal toxicity.3
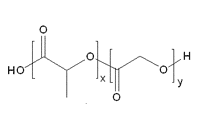
Figure 3. General Structure of PLA: PGA copolymer
Poly (Lactide-co-Glycolide) Copolymers (PLGA)
Among the co-polyesters investigated, extensive research has been performed in developing a full range of PLGA polymers. Both L- and DL-lactides have been used for co-polymerization. The ratio of glycolide to lactide at different compositions allows control of the degree of crystallinity of the polymers.4 When the crystalline PGA is co-polymerized with PLA, the degree of crystallinity is reduced and as a result this leads to increases in rates of hydration and hydrolysis. It can therefore be concluded that the degradation time of the copolymer is related to the ratio of monomers used in synthesis. In general, the higher the content of glycolide, the quicker the rate of degradation. However, an exception to this rule is the 50:50 ratio of PGA: PLA, which exhibits the fastest degradation.5,6
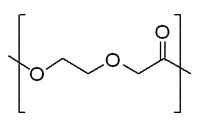
Figure 4. General Structure of Polydioxanone
Polydioxanone (PDS)
Although biodegradable polylactides and glycolides have been used to develop versatile resorbable multi-filament structures, there is growing research involved in the development of materials that form monofilament sutures. Multifilament sutures have a higher risk of infection associated with their use and cause a greater amount of friction when penetrating tissues.7,8 Polydioxanone (referred to as PDS) is made by a ring-opening polymerization of thep-dioxanone monomer. It is characterized by a glass transition temperature in the range of –10 to 0 °C and a degree of crystallinity of about 55%. Materials prepared with PDS show enhanced flexibility due to the presence of an ether oxygen within the backbone of the polymer chain. When used in vivo, it degrades into monomers with low toxicity and also has a lower modulus than PLA or PGA. For the production of sutures, PDS is generally extruded into fibers at the lowest possible temperature, in order to avoid its spontaneous depolymerization back to the monomer.
End Groups
The RESOMER® materials offered in our catalog are available with three types of end-groups functions: (i) free carboxylic acid group, (ii) ester terminated group, (iii) alkyl ester group. Polymers capped with ester terminated and alkyl ester groups typically show longer degradation lifetimes than the free carboxylic analogs. 9 This choice in end group provides a range of PLA/PGA materials with distinctive properties to suit your individual research needs.
Quality and Specifications of RESOMER Polymers
All RESOMER® polymers are made by Evonik Röhm GmbH and will conform to a rigid set of pre-determined specifications in order to ensure that the material has consistent properties for the entire process, from research through commercialization. Examples of specifications that you may find useful include:
- Residual Monomers
- Inherent Viscosity
- Heavy Metal Content
- Sulfated Ash Content
In addition to the new RESOMER® products, we offer an extensive line of other synthetic biodegradable and natural polymers.
References
To continue reading please sign in or create an account.
Don't Have An Account?