Development of Organic Semiconductors
Introduction
The soaring global demand for energy, coupled with the limited supply of fossil fuels, has increased the need for renewable, low-cost energy sources. Organic electronics have shown great promise for applications in lighting, power, and circuitry, with rapidly improving performance already surpassing that of amorphous silicon-based counterparts.1,2 Devices designed with organic electronics semiconductors are of relatively low cost because their solution-processable active layers can be printed using traditional textile methods.
The synthesis of new electronically active materials is driving the advances in this burgeoning field. These pi-conjugated systems, including heteroacenes and polythiophenes, are the foundation of organic semiconductor research.3,4 Thiophenes, oligothiophenes, and polythiophenes have been widely studied in the development of organic electronics; the synthetic accessibility of these molecules and their derivatives have been explored by chemists around the world (Figure 1).5,6 These investigations have led to unprecedented growth in the performance of thiophene-based photovoltaics and organic fieldeffect transistors (OFETs).7,8
Synthesis of Oligothiophenes and Polythiophenes
Since substituted polythiophene was first synthesized in 1980, there has been growing interest due to its favorable chemical and physical properties, such as high conductivity, and environmental and thermal stability. However, challenges in processing limited its utility until polythiophenes with flexible side chains were prepared, which improved solubility.9,10 These early methods carried out solely by chemical and electrochemical strategies produced undesirable head-tohead (HH) and tail-to-tail (TT) couplings, which resulted in a sterically twisted structure in the polymer backbone. This, in turn, affected the thin film microstructure and resulted in poor device performance. In 1992, McCullough et al. developed synthetic methods to afford regioregular Poly(3-alkylthiophene) (rrP3AT) with a HT regioregularity of 98–100%.11 An alternate route developed by Rieke utilized highly reactive “Rieke® zinc“ (ZN*) to prepare regioregular P3ATs. In 1999, the Grignard metathesis (GRIM) method was reported as an economical route to prepare rrP3ATs with the high regioregularity of >99% HT couplings.11 The GRIM method also offered rapid and easy preparation in large scales under mild reaction conditions. These discoveries brought about not only the development of a wide variety of welldefined polythiophenes, but also a dramatic enhancement in the electrical properties of rrP3ATs, due to planarization of the backbone and solid-state self-assembly to form well-defined, highly organized three-dimensional polycrystalline structures. The synthetic methods are provided in Scheme 1. These structures provide efficient intermolecular interactions and supramolecular ordering of the polymer backbone and side chain in the solid state, which lead to high mobility.
a) X for intermediate, 2 is Br (not H) in this case. b) R’ = Alkyl, X’ = Cl, Br
Scheme 1. Synthetic methods for the preparation of regioregular poly(3-alkylthiophene)s.11 Reprinted with permission from ACS.
Another popular method for producing polythiophenes is via palladium-catalyzed cross-coupling reactions (Stille and Suzuki). By choosing different building blocks, a multitude of molecular architectures have been produced, allowing tuning of the electronic properties over a wide range. However, the Stille and Suzuki polymerization methods have limited control over molecular weight and polydispersity.11
In parallel to the remarkable developments surrounding polythiophenes for organic semiconductor materials, oligothiophenes were also realized as promising active semiconductor materials in OFETs. Furthermore, structurally defined, monodisperse oligothiophenes are excellent model compounds for establishing valuable structure-property relationships and extrapolations to their polymer analogs. Although the synthesis of oliothiophenes involves multiple steps, these defect-free materials are easy to modify by different functional groups, which can provide novel properties to the pi-conjugated core system. Therefore, functionalized oligothiophenes have been considered as a third generation of advanced conjugated materials for organic electronic devices.7
Electronic Devices Based on Single Crystals of Oligo- and Polythiophenes
Some of the first devices fabricated from single crystals of a poly(3-hexylthiophene) (P3HT) (Prod. No. 698989 and 698997) were produced by Cho and coworkers in 2006.12 They crystallized P3HT from solution onto a self-assembled monolayer (SAM) of silane-treated silicon via a “self-seeding process.” This process involved pouring a supersaturated solution of P3HT onto the SAM-modified substrate, resulting in P3HT microwires. Transistors were subsequently fabricated from these microcrystals, using the SAM as a dielectric. The crystal packing showed the 1-D crystal exhibited pi-pi stacking along the long axis with an intermolecular distance of 3.9 Å. Cho and coworkers expanded upon this work by preparing single crystals of P3HT and poly (3-octylthiophene) (P3OT) via solvent vapor annealing and slow, controlled crystallization, respectively.13 Single crystal transistors with excellent carrier mobilities were reported for both P3HT and P3OT (Prod. No. 682799). The results of this study are shown in Figure 2.
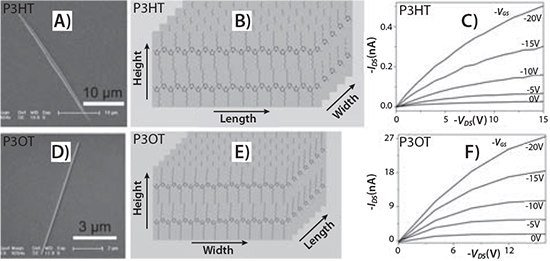
Figure 2. A) TEM image of a P3HT crystal. B) Schematic illustration of the crystal structure of P3HT. C) Output characteristic of FETs based on a P3HT crystal. D) TEM image of a P3OTcrystal. E) Schematic illustration of the crystal structure of P3OT. F) Output characteristic of FETs based on a P3OT crystal.13
Polythiophenes are among the most studied polymers for organic photovoltaics (OPVs). However, they are generally investigated as thin films, where grain boundaries limit their charge transport. The disordered, amorphous regions between crystallites reduce the short-circuit current (Jsc) in solar cells, thus limiting their overall efficiency. One common method for overcoming this limited crystallinity is via thermal annealing, which increases the size of the crystallites, thus improving the device performance. Single crystals naturally exhibit better charge transport in OPVs because they do not contain defects which reduce performance. Utilizing this concept, the Jenekhe group designed photovoltaics containing single-crystalline nanowires of poly (3-butylthiophene) (P3BT)14 (Prod. No. 495336 and 511420). Nanowires, crystallized by slowly cooling from solution, were mixed with PCBM, a common electron acceptor (Figure 3). This mixture was then spin-coated onto indium-tin oxide (ITO) doped glass coated with a holeconducting layer of PEDOT:PSS. The resulting nanowire network in the active layer of the photovoltaic device was characterized via TEM and AFM, which showed long, thin nanowires with diameters of 8–10 nm and lengths up to 10 μm. Utilizing these materials, solar cells converted light to electrical energy with an efficiency of ~3%, an order of magnitude greater than devices created using polycrystalline thin films of P3BT/PCBM. The nanowires eliminated many trap states in the electron-donating P3BT, lowering the HOMO orbitals, as evidenced by the red-shift in UV-Vis absorption, and slightly increased the open-circuit voltage (Voc). Additionally, the percolating network allowed efficient extraction of charges, resulting in a better fill factor (FF) and increasing the short-circuit current (Jsc) by 57%.
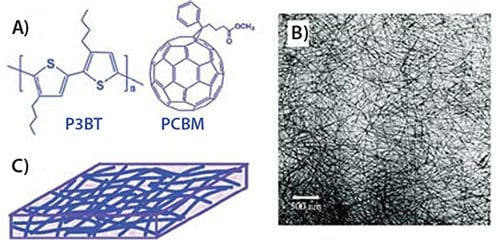
Figure 3. A) Chemical structures of P3BT and C61-PCBM. B) TEM images of P3BT-nw/C61-PCBM (1:1 wt% ratio) nanocomposites. C) Schematic illustration of nanowire network of P3BT/PCBM composites.
Fundamental research into the crystallization and assembly of organic-inorganic hybrid p-n junctions has also been conducted by Briseno et al. End-functionalized P3HT and dodecyquaterthiophene (QT) were end functionalized with phosphonic acid and subsequently grafted onto zinc oxide (ZnO) nanowires, which resulted in the creation of core-shell p-n junction hybrid nanowires.15 The crystallographic features of this system were explored via TEM; paralleling their thin-film counterparts, the P3HT shells were less crystalline and exhibited some amorphous domains (Figure 4). In contrast, the oligomers selfassembled into single crystals, exhibiting multiple levels of intermolecular interactions, including hydrogen-bonding, van der Waals forces, and pi-pi interactions (Figure 5).
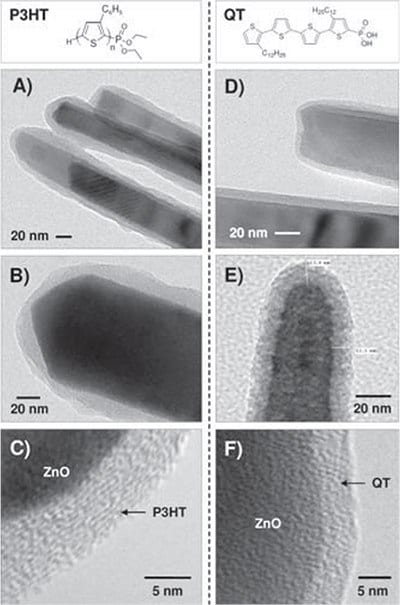
Figure 4. A-C) Transmission electron microscopy (TEM) images of ZnO/P3HT core-shell nanowires and D-F) ZnO/QT core-shell nanowires.
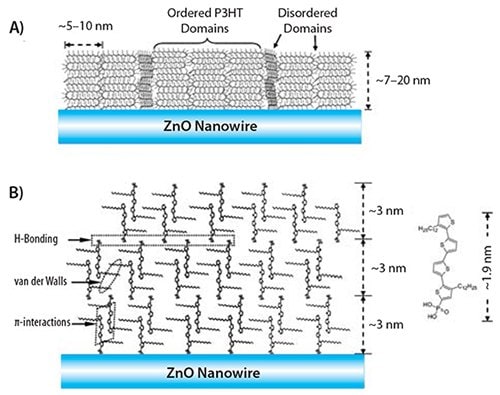
Figure 5. A) Diagram of the ZnO/P3HT interface. B) Molecular packing at the ZnO/QT nanowire interface. Also shown are the three molecular forces that drive interfacial selfassembly.
Another demonstration of ordered self-assembly on the nanometer scale was recently presented by Lee et al.16 In that study, the morphological structure of a polythiophene amphiphilic diblock copolymer which contained nonpolar hexyl and polar triethylene glycol side chains was investigated. While chloroform was a good solvent for both blocks, P3HT crystallized into nanowires upon the addition of a nonsolvent, such as methanol. However, the hydrophilic polythiophene block containing a triethylene glycol side chain was soluble in methanol, and therefore the diblock copolymer formed crystalline aggregates when methanol was added to the chloroform solution. Furthermore, the addition of a salt, such as potassium iodide (KI), drove self-assembly at larger scales in this system. When KI and methanol were added concurrently to a solution of the diblock copolymer in chloroform, superhelical nanowires were observed. Helices resulted from the complexation of the potassium cations by the triethylene glycol side chains (Figure 6).
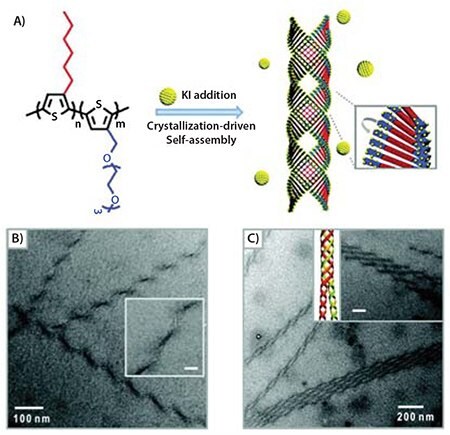
Figure 6. A) Molecular structure of P3HT-b-P3(TEG)T diblock copolymers and schematic representation of their assembly into superhelical structures through crystallization in the presence of potassium ions. B) TEM images of copolymer after addition of KI revealed helical ribbons with a regular pitch. Inset: magnified image (scale bar: 20 nm). C) TEM image of multiple-stranded helices. Inset: TEM image and schematic showing association of double helices into quadruple superhelices (scale bar: 100 nm).
Conclusion
Thiophene-based materials will undoubtedly continue to play a large role in the development of organic electronics. The versatility of these oligomers and polymers will continue to be explored through developments in new synthetic methods and derivatives in order to optimize their molecular, electronic, and morphological properties for increased performance and efficiency. Furthermore, new processing techniques will increase crystallinity, and thus improve performance of the devices based upon these remarkable materials. Single crystals offer the opportunity to study the intrinsic properties of these molecular semiconductors in the solid state and fabricate devices that are not limited in performance by grain boundaries or other morphological defects.
Materials
References
To continue reading please sign in or create an account.
Don't Have An Account?