Alternative Energy Tutorial
Introduction
Batteries
Fuel Cells
Conducting Polymers
High-Purity Inorganics
Liquid Electrolytes
Plasticizers and Binders
Solid Polymeric Electrolytes
Related Products
References
Introduction
Fuel cells and batteries are electrochemical cells used to generate an external electrical current. They consist of an anode, where oxidation occurs, a cathode, where reduction occurs, and an electrolyte through which ions can travel between electrodes (see Figure 1 for a schematic of an electrochemical cell). In fuel cells (discussed below), one or both of the reactants are supplied from an external source to the cell. Though technically fuel cells, when the only reactant supplied to the cell is atmospheric oxygen, the cells are considered batteries (zinc/air or aluminum/air cells for example).
Batteries
Batteries can be divided into two types: primary or disposable batteries and secondary or rechargeable batteries. The main advantages of batteries over fuel cells are their availability, portability, low cost, and wide range of operating conditions. Batteries, however, have much shorter life spans and lack the power output of fuel cells. Power outputs of batteries are typically on the order of 100's of watts, whereas fuel cells can provide kilowatt to megawatt outputs, power enough to light a building or fuel a vehicle for hours. Under heavy energy demands, batteries can build up dangerous levels of heat and pressure, degrading the battery and possibly causing leaks of toxic compounds or even explosions. In addition, the limited life of primary batteries and the limited cycle life (number of times it can be recharged) of most secondary batteries necessitates the need for disposal of often dangerous and toxic battery materials. Table 1 summarizes some of the common types of primary and secondary batteries.
The primary component materials of a battery are the anode, cathode, electrolyte, and semi-permeable materials. In addition various catalysts have been used to enhance the performance of electrodes. For example, ruthenium(IV) oxide (Prod. No. 238058) is used as a catalyst in a vanadium redox battery system.1 Table 1 summarizes some of the types of electrodes and electrolytes used in common batteries. Many advanced battery designs focus upon new materials for these key components.
Much of the recent battery work has focused on lithium-ion batteries, since they are the primary power source for the ever-growing field of small, rechargeable electronic devices. Nickel sulfide, for example, was recently explored as a cathode material for rechargeable lithium batteries.2 Current research is also concerned with some very mundane materials in electrodes. New morphologies of graphite flakes (Prod. No. 332461), as a case in point, have been studied as anode material in lithium-ion batteries.3 Electrolytes are also very important in battery performance. An LiBF4 (Prod. No. 451622) solution, for example in a butyrolactone/ethylene carbonate (Prod. Nos. B103608 and E26258) solution has proven to be a highly conductive and highly thermally stable electrolyte for lithium-ion batteries.4
Fuel Cells
Fuel cells offer the promise of a clean energy source for stationary power generation. They produce energy from hydrogen, natural gas, alcohol, or other readily available hydrocarbon fuels (Figure 2). Fuel cells date back to the nineteenth century when Grove, in 1839, first published his work on the generation of electricity by partially immersing two platinum electrodes and separately supplying oxygen and hydrogen to them.5 There is considerable current interest in fuel cells as an environmentally clean alternative to fossil-fuel-burning power sources.
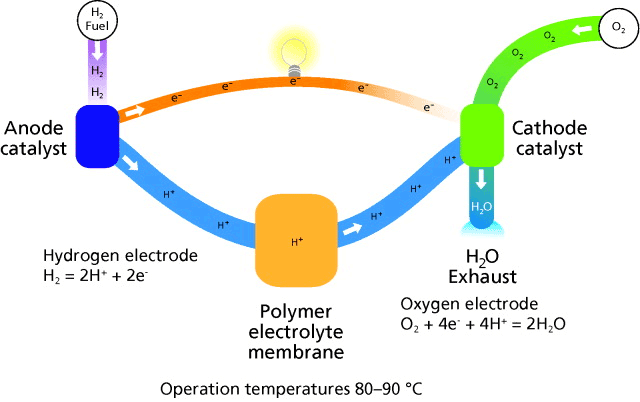
Figure 2.Schematic of a typical polymeric electrolyte membrane (PEM) fuel cell.
Purely fuel cell powered vehicles are currently being tested as prototypes with plans for eventual commercialization by as early as 2010.6 Some of these vehicles are already in operation in municipal organizations around the world. See Table 1 for some examples.

Figure 3.Oxidation/Reduction reaction in a fuel cell.
The hydrogen used in fuel cells can be supplied directly or indirectly from a fuel reformer that converts alcohol, natural gas, or other hydrocarbon fuels into hydrogen. Since the primary exhaust of fuels cells is water, they offer the promise of an environmentally friendly power source.
Table 3 provides a comparison of some currently available fuel cells. In addition, some fuel cells currently under development include:
- Regenerative fuel cells. In these cells, water is converted to hydrogen and oxygen by a solar-powered electrolyzer. The cell then coverts this fuel into electricity, heat, and water. The water is then recycled into the electrolyzer. NASA is currently spearheading this technology.
- Zinc-air fuel cells (ZAFC). In ZAFCs, atmospheric oxygen is passed through a gas diffusion electrode and converted to water and hydroxyl ions. The ions then travel through an electrolyte to a zinc anode where it reacts to form zinc and electricity. Much like a rechargeable battery, the zinc electrode can be regenerated. Unlike a battery, however, this process takes only about five minutes.
- Protonic Ceramic Fuel Cells (PCFC). These cells use high operating temperatures (700 °C) and ceramic electrolytes with high protonic conductivity. As a high temperature cell they have similar advantages to MCFCs while also integrating many of the advantages of PAFCs such as high proton conduction. In addition, they electrochemically oxidize fossil fuels at the anode, eliminating a hydrogen producing step.
Fuel cells have the same basic components as batteries: anode, cathode, and electrolyte. Yttria-stabilized zirconia (Prod. Nos. 572349 and 572322) is one of the most commonly used electrolytes in solid oxide fuel cells.5 Lanthanide-doped ceria (Prod. Nos. 572330, 572357, 572365, or 572381) is also gaining favor as a fuel cell electrolyte due to its improved properties at lower temperatures.8 Furthermore, doped cerium oxide electrolytes exhibit an ionic conductivity three to five times greater than that of yttria stabilized zirconia. Platinum black (Prod. No. 205915) and platinum-based alloys are the most common electrodes for fuel cells. Recent work by Scherer and coworkers have recently developed a good model to examine the surface area effects of platinum electrodes as well as glassy carbon (Prod. No. 484164) electrodes.9
Catalysts for Fuel Cells
Platinum is the most common catalyst for fuel cells, however, due to its high cost it is often doped with palladium, ruthenium, cobalt/nitrogen complexes, or more recently iridium or osmium. In addition to its high cost, platinum is also quite rare. In fact, there is not enough platinum in the world to equip every vehicle in use today with a traditional (Pt-catalyst) proton exchange membrane (PEM) fuel cell. For this reason, new catalysts, doped-platinum catalysts, and new platinum-deposition techniques are all being developed to reduce the amount of platinum needed for fuel cell catalysts.
Conducting Polymers
The discovery over twenty five years ago of relatively high electrical conductivity (~10+3 S/cm) of doped polyacetylene10 sparked extensive research in the application of conjugated polymers in such diverse fields as electronics, energy storage, catalysis, chemical sensing, biochemistry, and corrosion control.11,12 Unfortunately, the conducting polymers were found to be unstable in air and difficult to process. Significant advances in improving the desired electrical, optical, and mechanical properties, while simultaneously enhancing processability and stability, have been realized by cross-disciplinary collaborations between chemists, physicists, materials scientists, and engineers.
Polyaniline is becoming the conducting polymer of choice in many applications for several reasons: its electronic properties are readily customized, it exhibits excellent chemical stability, and is the least expensive of the conducting polymers.
Polythiophenes have been studied extensively for use in light emitting diodes, among other applications, due to the chemical variability offered by substitution at the 3- and 4- positions. The regularity of the side-chain incorporation strongly affects the electronic band gap of the conjugated main chain and is critical to device performance.13 We offer highly regiocontrolled alkylsubstituted polythiophenes (P3HT): almost completely regioregular head-to-tail (HT) P3HT (Prod. Nos. 698997 and 698989) and regiorandom (1:1 HT/HH) P3HT (Prod. No. 510823).14
High-Purity Inorganics
Wemaintain the highest standards for quality control and quality assurance. Our high-purity materials are rigorously analyzed by a variety of techniques including trace metals analysis by ICP, which can detect impurities an order of magnitude below ppm levels. Fuel cells and batteries often require high-purity components. For example, the electrolytes in low-temperature rechargeable batteries can be from alkyl carbonates and high purity lithium salts such as LiPF6 (Prod. No. 450227) and LiAsF6 (Prod. No. 308315).15
High-purity inorganics also find significant industrial usage. More than 60% of the industrially used cadmium is in Ni-Cd batteries, of which 75% is found in cellular phones. Much of the remainder of this portion is also used in the telecommunications industry as materials in emergency power supplies for electronic telephone exchanges.
Liquid Electrolytes
The type of electrolyte used for a fuel cell depends upon the choice of fuel cell (see Table 2). The key role of the electrolyte is to create a medium through which ions can move between the anode and the cathode. Electrolytes can also act as a kind of filter, preventing undesirable ions or electrons from disrupting the desired chemical reactions.
Plasticizers and Binders
The use of plasticizers in commercial polymer formulations to decrease Tg and the internal viscosity, and to increase bulk flexibility is a well-established practice in a multitude of industrial applications. In fact, the "new car smell" enjoyed by many car owners results mainly from the phthalate plasticizer vaporized in the closed car interior, and actually advertises the deterioration of the vinyl upholstery. To improve the permanence of the plasticizer higher-molecular-weight phthalates are commonly used for modern car interiors. A number of criteria are considered in choosing a plasticizer, including cost, compatibility, stability, ease of processing, and permanence. In addition to the aforementioned uses, a growing body of research has emerged over the past two decades on the application of plasticized polymers in areas that involve properties not usually associated with polymers. For example, the introduction of oligomeric poly(ethylene glycols) (PEG) and derivatives as plasticizers, to effect a significant increase in ionic conductivity as solid polymer electrolytes (SPEs), for use in high energy density batteries and other solid-state electrochemical devices.16-18
Cellulose triacetate membranes, plasticized with 2-nitrophenyl octyl ether (Prod. No. 365130), are used as materials for separations. They are impermeable to metal cations, but allow anion exchange19 and are also remarkably permeable to neutral, mono- and disaccharides.20 Highly efficient photorefractive polymer composites can be formed using 9-ethylcarbazole (Prod. No. E16600) (ECZ) as a plasticizer in guest-host polymers.21
Solid Polymeric Electrolytes
NASA's jet propulsion laboratory is currently investigating SPEs formed by reacting lithium salts (e.g. LiClO4, LiPF4, and LiCF3SO3) with cyanoresins for rechargeable batteries and electrochemical cells. Specifically, SPEs would be used as separators between carbon composite anodes and cathodes. It has been proposed that these batteries would have an energy density of 80 W·h·lb-1 and be viable for 1000 recharge cycles. Polyacrylonitrile (Prod. No. 181315), polyvinyl pyrrolidone (Prod. Nos. 234257 and 856568), and polyethylene (Prod. No. 427772) have dielectric constants between 4 and 5 and lithium-ion conductivities between 10-6 and 10-5 S·cm-1. Unfortunately these room-temperature conductivities are too low for effective power generation. SPE's formed from amorphous cyanoresins such as cyanoethyl polyvinyl alcohol (CRV), cyanoethyl pullulan (CRS), and cyanoethyl sucrose (CRU) have dielectric constants as high as 20 or more and lithium-ion conductivities 100 times greater than conventional SPEs.15
We also carry a complete line of Nafion® resins. Nafion® resins are perfluorinated ion-exchange materials composed of carbonfluorine backbone chains and perfluoro side chains containing sulfonic acid groups. Solid polymer fuel cells for pulse power delivery are based on Nafion® solid polymeric electrolytes.22
References
To continue reading please sign in or create an account.
Don't Have An Account?