Light-emitting Polymers
Introduction
Conjugated polymers with long range p-electron delocalization behave as processable organic “metals” in their doped state and as semiconducting materials in their neutral undoped state. Many undoped polymers exhibit strong photoluminescence (PL) in the visible and near infrared range. Switching between doped and undoped states induces changes in a number of Light Emitting Polymer (LEP) properties, such as polymer volume, absorption color, and reversible PL quenching. These controlled changes make LEPs promising for applications: an induced variation in absorption color may be exploited for electrochromic displays while a change in volume may be utilized for electroactive artificial polymer muscles. The combination of semiconductivity and intense PL results in LEP electroluminescence and their use in polymer light emitting diodes (PLEDs). The high sensitivity of PL quenching to doping or charge transfer can be used to detect biological and explosive species. Therefore, the LEPs represent an important category of low-temperature processable materials useful for many scientific and technological explorations.
PLEDs are currently under development for applications in flat panel displays and lighting with strong commercialization potential that depends on understanding and improvement of properties of the LEPs. For example, although a PLED has a relatively simple thin-film device structure as illustrated in Figure 1, a high-performance PLED requires the LEP layer to meet several stringent requirements: (1) color purity, which is determined by the polymer bandgap and film morphology; (2) matching of ionization potentials and electron affinities between LEP and the different electrode materials; (3) high PL quantum efficiency; (4) chemical and thermal stability; and (5) processability which involves solubility, solution viscosity, and solvent-substrate compatibility. These properties can be adjusted by changing the chemical structure of the conjugated polymer chains, side groups, incorporation of heteroatoms, molecular weight, structural regularity, and/or copolymerization. This article reviews the main categories of LEPs tailored for different applications. Note that LEPs include both polymers containing fully conjugated main chain and those with conjugated segments in the main chain or side groups. Most conjugated oligomers exhibit similar processability and luminescent properties as their polymeric counterparts.
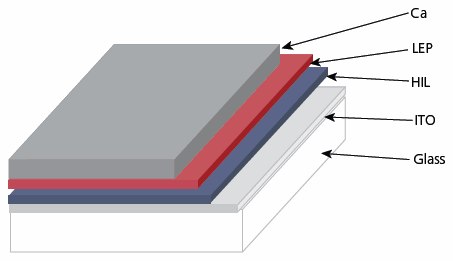
Figure 1.Schematic illustration of a polymer light emitting diode (PLED). HIL=hole injection layer, usually a spin-cast film of an inherently conductive polymer (PEDOT or polyaniline).
Poly(1,4-phenylene vinylene) (PPV)
References to polymer structures depicted in Figure 2 are shown in boldface. PPV (1) and its soluble derivatives are among the most widely studied LEPs. PPV is generally prepared via a precursor, poly(xylylidenetetrahydrothiophenium chloride), which is soluble in water and methanol. PPV was used to make the earliest conjugated polymer light emitting diode (LED), but the relatively low PL quantum efficiency and high-temperature conversion of the precursor to PPV prompted the synthesis of many new PPV derivatives that are soluble in common organic solvents. MEH-PPV (Product No. 541443) can be conveniently synthesized from the corresponding monomer 1,4-bischloromethyl-2-methoxy-5-(2’-ethylhexyloxy)benzene (Scheme 1a). The dialkoxy side groups also modify the polymer’s bandgap, so that the emission color bathochromically shifts to orange from the yellow-green of the unsubstituted PPV. MEH-PPV was used in the fabrication of first high efficiency polymer LEDs. The emission color is slightly shifted further toward red when the 2’-ethylhexyloxy side group is replaced by 3’,7’-dimethyloctyloxy group (MDMO-PPV). In BCHA-PPV(3) where the side groups are bulky 2,5- bis(cholestanoxy), the color is shifted in the opposite direction, to orange-yellow. Many other side groups have also been used to modify the emission color. Side groups can also be introduced to the vinyl moieties of PPV. For instance, poly[(1,4- phenylene-1,2-diphenylvinylene)] (4) is a green emitter with high PL efficiency and stability.
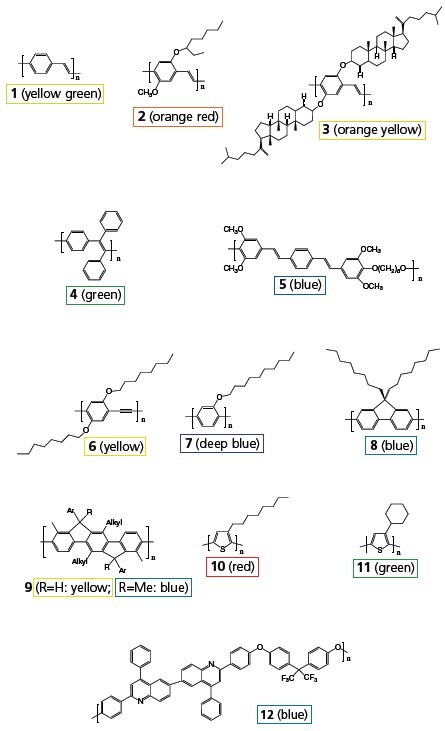
Figure 2.Representative classes of light-emitting polymers referenced in the following paragraphs.
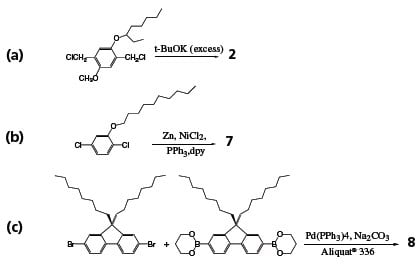
Scheme 1.Synthetic routes to polymers (2), (7), and (8).
Gilch polymerization was used to prepare MEH-PPVs in high yield and high molecular weight. In the Gilch reaction of 1,4-bischloromethyl-2,5-bis(3’,7’-dimethyloctyloxy)benzene, 1.5–2.0% of tolane-bisbenzyl moieties were found to be the only defects on the PPV mainchain. In the polymerization of 1,4-bischloromethyl-2-methoxy-5-(3- decyloxyphenyl)benzene, the p-quinodimethane intermediate is fairly polar. The subsequent free-radical polymerization is almost exclusively a head-to-tail coupling that yields a substituted PPV containing 0.5% of the tolane-bisbenzyl defects. Such low defects are important for achieving high performance polymer LEDs. Substituted PPV with relatively low molecular weights can also be synthesized by condensation polymerization routes (Wittig, Heck, Knoevenagel). In the Knoevenagel polymers, a cyano group is introduced to each vinylene moiety of dialkoxy-PPV. The resulting polymers show an intra molecular “push–pull” effect of π-electron clouds that effectively reduces the bandgap and shifts the emission color into deep red region.
The emission color and the PL quantum efficiency of substituted PPV can be finessed via copolymers of PPV containing different side groups. For instance, varying the ratio of the comonomers in poly[(2-dimethyloctylsilyl-1,4-phenylene vinylene)-co-(MEH-PPV)], can systematically tune the emission color from green of the silyl-PPV to orange in MEH-PPV. To obtain emission colors in the green to blue range, one may adopt copolymers consisting of short segments of PPV strung together with non-conjugated moieties such as alkylenedioxy, oligo(ethylene oxide), and dimethylsilane. Copolymer (5) is an efficient blue emitter. These copolymers, however, generally exhibit poor charge carrier conductivity. Poly(2,5-dialkoxy-1,4- phenyleneethynylene) (PPE) are dehydro analogs of dialkoxy- PPV. The emission peak of the PPE (6) (Product No. 659894) is narrower and blue shifted compared to corresponding PPVs.
Poly(1,4-phenylene) (PPP)
Blue LEPs can made from poly(1,4-phenylene) (PPP) and its various derivatives. Soluble PPPs cane be synthesized from the corresponding dichloro, dibromo or diborate monomers via Grignard, Ni0-catalyzed Yamamoto, or Suzuki reactions. The molecular weights of the resulting polymers, however are rather low, often less than 10,000. High molecular weight soluble PPPs containing electron-withdrawing groups such as benzoyl, can be obtained via the Ni0-catalyzed Yamamoto coupling of the corresponding dichloro-monomers (Scheme 1b). All of the substituted PPP emit deep blue light with a significant portion of the emission in the UV region. Poly(2-decyloxy-1,4-phenylene) (7) exhibits both high PL and EL quantum yields. The emission peaks at about 410 nm.
Polyfluorenes (PFO)
Polyfluorene, in which every pair of phenyl rings are locked in plane by the C-9, has a slightly smaller bandgap than PPP. Most of its PL is within the blue region of the visible spectrum. In poly(9,9-dioctylfluorene) (8) (Product No. 571652), the solubilizing alkyl groups are located on the C-9, far away from the C-2 and C-7 positions and interfere little with the polymerization of the corresponding monomers that links the fluorene units through C-2 and C-7. Polymers with Mw > 100,000 and PL quantum yield > 70% have been obtained by both the Ni0-catalyzed Yamamoto coupling and the Pd0- catalyzed Suzuki coupling polymerization. With the use of a surfactant (for example, Aliquat® 336) to increase the mixing of solvents in the Suzuki polymerization (Scheme 1c), the molecular weights can be further increased up to 1,000,000. Alternating PF copolymers can be synthesized by the Suzuki route. Introduction of triphenylamine comonomers enhances the hole-transporting ability of the polyfluorenes, whereas thiophene and 1,3,4-benzothiadiazole comonomers reduce the polyfluorene’s bandgap and shift the emission color toward green or even red. Soluble derivatives of polyfluorenes and PPVs are currently commercialized for LED display and illumination products.
Further locking of the phenyl rings into its coplanar structure is obtained in ladder-type PPPs. The enhanced conjugation along the polymer backbone and eximer formation due to interchain interaction entails large side groups for solubility. The ladder polymer (9, R=H) exhibits intense blue luminescence in dilute solution. The PL of the solid thin film shifts to yellow with only 10% quantum yield due to eximer formation. When R is replaced with a methyl group, the eximer formation is suppressed, and the resulting solid thin film exhibits intense blue luminescence, similar to the polymer in dilute solution.
Poly(thiophenes)
Poly(3-alkylthiophene)s have been extensively studied for their thermo- and solvatochromism, as well as for applications in field effect transistors. Regiorandom poly(3-octylthiophene) (10) shows relatively weak red PL in dilute solution. The emission is largely quenched in concentrated solutions and solid thin films. Bulky side groups such as cyclohexyl (11) twist the coplanarity of the polythiophene main chain and shift the emission color to green. Poly(3-methyl- 4-cyclohexylthiophene) emits blue light. On the other hand, regioregular poly(3-alkylthiophene) is shown to have coplanar polymer main chains that can orderly pack into crystalline nanometer-size domains with high hole mobility. They are being studied as p-type semiconductors for thin film transistors and solar cells. Thiophene, 3- alkylthiophene, and 3-alkoxythiophene are frequently used as comonomers to reduce the bandgap of PPP, PF, and PPV.
Nitrogen-Containing Polymers
Heterocyclic rings containing imino-N are electron-acceptors when conjugated with hydrocarbon-based π-systems. Poly(2,5-pyridinevinylene) emits red light in dilute solutions, but PL quantum efficiency is relatively low due to strong dipole interaction that promotes aggregate formation. Protonation or alkylation of the N-atom causes a complicated change of emission color and efficiency. Quinoline is a useful building unit for PPP-type copolymers with high electron affinity. Polyquinoline (12) is an efficient blue emitter. 1,3,4-Oxadiazole is another heterocyclic aromatic ring often copolymerized with phenyl rings to increase electron affinity. 1,3,4-Oxadiazole containing polymers and copolymers with large bandgap are often used as electron-transporting materials, while those with smaller bandgap and visible light emission are also used in PLEDs. On the other hand, tertiary amine and derivatives are used as hole transporting polymers. Poly(9-vinylcarbazole) (PVK) (Product No. 182605) is a good photoconductive material. It is a popular wide-bandgap host for other emissive materials such as perylene and phosphorescent dopants.
Water-Soluble LEPs
Ionic groups such as quaternary ammonium and sulfonate can be attached to conjugated polymers via a flexible tether to render solubility in water or methanol. The sulfonatosubstituted polythiophene is self-dopable in water and does not show appreciable luminescence upon doping. The sulfonated PPV (Product No. 659894), PPP, and PF exhibit intense PL in dilute water solution with emission color similar to analogous polymers without the sulfonato groups. These water-soluble luminescent polymers have been studied for biosensing due to the high sensitivity quenching of the PL by electron-acceptors like methyl viologen (Product No. 856177).
Conclusion
Light-emitting polymers are characterized by (1) a high absorption coefficient, as high as 105/cm, (2) a high PL efficiency, since a quantum efficiency greater than 50% is frequently obtained for blue and green emissive polymers in solid state thin films, and (3) a large Stokes’ shift and thus show minimal self absorption of its PL emission. Polymer synthesis provides a convenient tool to tune these properties: Figure 3 illustrates LEPs that cover the entire visible spectrum. The LEPs enable a wide range of applications, including sensors, flexible LED displays and lighting devices, optical pump lasers, and potentially polymer diode lasers. It is important to note that susceptibility of the LEPs to environmental oxygen, moisture, and UV may present certain limitations and may inhibit the future commercialization of some of the products.
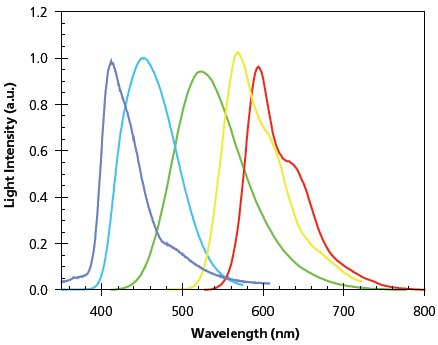
Figure 3.Film electroluminescence spectra of representative LEPs. Peak position from left to right: (7), (12), (4), (3) (2).
Materials
References
To continue reading please sign in or create an account.
Don't Have An Account?