Optoelectronic Devices with DPP-containing Molecules
Introduction
Optoelectronic devices such as light-emitting diodes (LEDs), solar cells, and light-emitting field effect transistors (FETs) that utilize organic materials as their light harvesting and/or charge transporting component have recently been the subject of much academic and commercial attention.1,2 This widespread interest is motivated by organic materials′ unique advantages compared to their inorganic counterparts, e.g., lowcost, lightweight, solution processable, and compatible with flexible substrates. Conjugated polymers are the most-widely investigated class of organic materials for these applications.3,4 Organic solar cells based on a conjugated polymers5 or small molecule/fullerene derivative blends have achieved a power conversion efficiency (PCE) up to 10%. In addition, FETs utilizing conjugated polymers as the charge transporting layer have demonstrated carrier mobilities up to 2.0–3.0 cm2V-1s-1 making them competitive with lower performing inorganic materials such as amorphous silicon.6,7 Conjugated small molecules have also historically been successfully used in FETs with pentacene and its soluble derivatives among the most studied such materials.8 Recently, a solar cell comprised of a soluble conjugated small molecule/fullerene blend achieved a PCE of 6.7%,9 narrowing the performance gap between organic solar cells made from conjugated polymers and conjugated small molecules. Compared to conjugated polymers, small molecules have several advantages such as well-defined structures, ease of synthesis and functionalization, no batch-to-batch variations, and the ability to use standard purification techniques.10 In addition, crystal structures of small molecules can be readily obtained by X-ray crystallography, providing an efficient approach to probe structureproperty relationships, which is essential for designing new materials.11,12

Figure 1.General molecular architectures of mono-DPP and bis-DPP small molecular compounds (upper) and four specific examples (mono-DPP: compounds 1-3; bis-DPP: compound 4 with different building blocks (bottom). D and A represent the electronic donating (D) and electronic accepting (A) properties that the building blocks may have.
A useful strategy for synthesizing conjugated small molecules for use in optoelectronic devices is to begin with a chromophore that can absorb most of the visible region of the solar spectrum. Diketopyrrolopyrrole (DPP) is such a chromophore and is also a desirable molecular building block because of its chemical stability, and ease of synthesis and modification. Figure 1 shows the general molecular architectures of DPP-containing small molecules synthesized in our lab. These compounds are classified into two subgroups: mono-DPPs which have only one DPP unit and bis-DPPs which have two DPP units. Due to the electron deficiency of the lactam structure in DPP, electron donating functional groups such as thienyl and phenyl derivatives are selected to incorporate with DPP as linkage and endcapping groups to further tune properties such as the optical band gap, highest occupied molecular orbital (HOMO), and lowest unoccupied molecular orbital (LUMO) levels. Alkyl chains are introduced on the conjugated backbone to induce solubility in common organic solvents, and to modify the degree of crystallinity and specific packing motif. Additionally, central groups in bis-DPP compounds provide a further structural handle capable of modifying material properties. This combination of different side chains and central, linkage, and endcapping groups allows the resultant material′s optoelectronic properties to be tuned with a high degree of sensitivity. This tunability enables engineering of the material properties necessary for optimal performance in optoelectronic devices. A number of DPP-containing derivatives have been synthesized by our group and several other groups.13-19 In Figure 1, we highlight several specific examples of DPP-containing conjugated small molecules for use in organic solar cells (compounds 1–3) and FETs (compound 4).
Synthesis
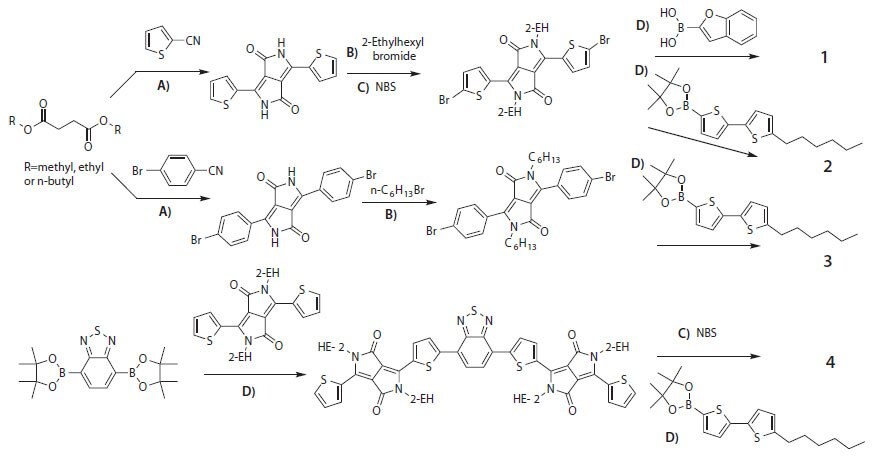
Scheme 1.Divergent synthetic routes of DPP-containing conjugated small molecules. A) Potassium t-butoxide, 2-methyl-2-butanol, reflux 5 h; B) K2CO3, DMF reflux 12 h; C) CHCl3, RT, 12 h; D) Pd2(dba)3, K3PO4, THF/H2O, 70 °C 12 h.
The DPP-containing small molecules displayed in Figure 1 are synthesized by the divergent approach illustrated in Scheme 1. For the mono-DPPs (compounds 1–3), this approach consists of several reaction steps such as DPP-linkage formation, N-alkylation, linkage bromination, and endcap group coupling. When the linkage group is a thienyl group, the DPP-thiophene can be prepared by a single reaction of a thienyl nitrile with a succinic diester. The resultant DPP-thiophene precursor is not soluble in organic solvents due to its internal hydrogen bonds. N-alkylation of the DPP-thiophene precursor with an alkyl bromide makes the precursor soluble enough for the subsequent modifications. The following bromination with N-bromosuccinimide (NBS) provides the thienyl groups with the reactive sites necessary to complete the final endcap group coupling. This final step can be achieved via a palladiumcatalyzed Suzuki or Stille coupling. When the linkage is a phenyl group, the above bromination step can be omitted by using bromobenzonitrile as the starting material. For the bis-DPPs, the divergent approach usually consists of iterative Suzuki or Stille couplings. Taking compound 4 as an example, an intermediate is synthesized by reacting an active benzothiadiazole (BT) precusor (benzothiadiazole-bis(boronic acid piancol ester)) with a mono-brominated DPP-thiophene via Suzuki coupling. The obtained intermediate is then brominated by NBS and subsequently reacted with an endcapping group via Suzuki coupling, generating compound 4. It is important to note the purity of the final material is critical for optimal device performance. It has been our experience that the presence of even a small amount of impurity can negatively affect the performance of a device. Therefore, both molecular design and precise purification are essential for synthesis of conjugated small molecules for high-performing optoelectronic devices.
Organic Bulk Heterojunction Solar Cells
In order for a material to be successfully used in a solar cell, its optical absorption profile must have a large overlap with the terrestrial solar spectrum. Figure 2A shows the pristine film absorption spectra of compounds 1–3. Their absorption edges extend to 670 nm, 704 nm and 775 nm, respectively. Due to its longer backbone conjugation length, compound 2 has a larger absorption edge than compound 1. Replacing the thienyl linkage with a phenyl linkage (compound 3) leads to a blue shift of approximately 105 nm. A twist conformation between the neighboring DPP and phenyl moieties has been confirmed from the single crystal structure of compound 3. This twist conformation reduces the effective conjugation length of the molecule and is likely the cause of this large blue shift in optical absorption.
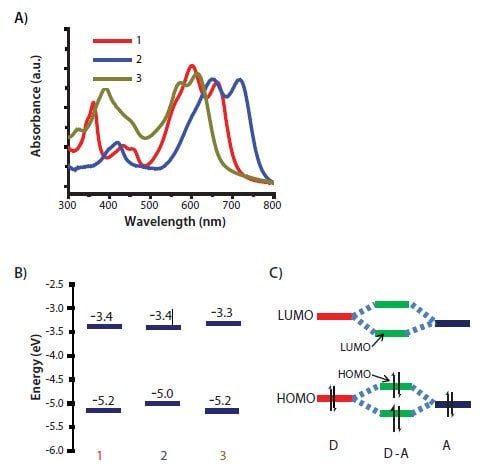
Figure 2.A) Normalized UV-Vis pristine film absorption and B) HOMO/LUMO energy levels of compounds 1-3. C) A simplified molecular orbital hybridization from donoracceptor moieties in a D-A molecule.
In addition to optical absorption, the HOMO and LUMO levels also play an important role in determining solar cell performance. Figure 2B shows the HOMO and LUMO energy diagrams of three compounds. The HOMO levels are determined by ultraviolet photoelectron spectroscopy (UPS) and the LUMO levels are calculated using the relationship LUMO = HOMO + Eg, where Eg is the optical energy bandgap estimated from UV-Vis film absorption cutoff. Compound 2 has the highest HOMO level (-5.0 eV) while the other two have the same value (-5.2 eV). Unlike the HOMO levels, the LUMO level of compound 3 is highest (-3.3 eV) while the others have a similar level (-3.4 eV). The differences among HOMO and LUMO levels can be explained using a simple model of molecular orbital hybridization as illustrated in Figure 2C. The DPP-containing compounds discussed here have a donor (D)–acceptor (A) or push-pull structure, where the DPP is the acceptor motif and thiophenes are the donor motif. The molecular orbital hybridization between the donor and acceptor motifs reduces the energy band gap of a D–A molecule. Furthermore, the HOMO and LUMO levels of a D–A molecule are dominated by the HOMO of the donor motif and the LUMO of the acceptor motif, respectively. Therefore, compound 2 has a higher HOMO value because it has a stronger donor motif (more thiophene rings). Compound 1 and 2 have the same LUMO value due to their use of the same acceptor motif. The twisted conformation between DPP and phenyl groups in compound 3 reduces D–A orbital hybridization and results in a deeper HOMO and a higher LUMO as compared with compound 2.
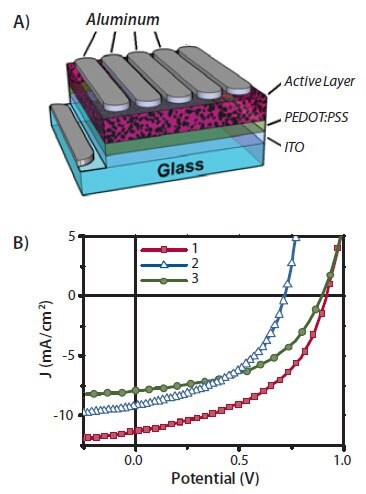
Figure 3.A) A schematic illustration of an organic solar cell device. B) Typical J-V curves of optimized solar cell devices fabricated using compounds 1-3 as donors with PC71BM as the acceptor (donor/acceptor mass ratio: 60:40).13-15,20
Solar cells using compounds 1–3 as donor materials were fabricated with a standard architecture of indium tin oxide (ITO)/ Poly(3,4- ethylenedioxythiophene) poly(styrenesulfonate) (PEDOT:PSS)/a DPPcontaining compound:[6,6]-phenyl C71-butyric acid methyl ester (PC71BM)/Al as shown in Figure 3A. Typical current density-voltage (J-V) curves of optimized devices under one sun irradiation (AM 1.5/100 mWcm-2) are presented in Figure 3B. The corresponding device performance parameters are listed in Table 1. Solar cell devices made from compound 1/PC71BM blends have the best performance (with a peak power conversion efficiency [PCE] of 4.8%) due to having high open circuit voltage (Voc) and short circuit current density (Jsc).15,20 Under low light intensity, a PCE of up to 5.2% can be achieved.21 Although the PCE value of the DPP material reported by our group in 2009 was lower as compared to some conjugated polymer solar cells, it has stirred a lot of interest in the community to pursue solution-processed small molecules as alternative donor materials in bulk heterojunction solar cells. Within two years, a new material having a PCE of 6.7% have been reported by Bazan and Heeger (UCSB)9 and the PCE of 10% reported by the Mitsubishi Chemical at the Fall 2011 Materials Research Society meeting in Boston is based on solution-processed small molecules.
In a bulk heterojunction solar cell, the Voc is usually determined by the HOMO level of the donor material (HOMOdonor) and the LUMO level of the acceptor material (LUMOacceptor), which can be described with the empirical equation Voc ≅ (1/e) (|HOMOdonor|-|LUMOacceptor|) - 0.3.22 Therefore, compounds 1 and 3 have a similar Voc because of their similar HOMO levels. The higher HOMO level of compound 2 results in a lower Voc. For the Jsc, the donor material that has a lower bandgap (larger absorption edge) theoretically would have a higher Jsc if similar charge recombination and trapping rates are assumed. This might be the reason for the higher Jsc of compound 1 compared to compound 3. Compound 2 has a lower Jsc than expected. We hypothesize this low Jsc might be the result of an undesirable film morphology and quality. Figure 4 shows the AFM height images of active layers in these optimized solar cell devices. The blend films of compounds 1/PC71BM and compound 3/PC71BM have similar morphologies which consist of rod-like crystalline domains with a good size distribution, while the compound 2/PC71BM blend film has a poor morphology with uneven domains and unclear boundaries. The root mean square roughness (RRMS) of these films highlighted in Figure 4 indicates a poor film quality of compound 2/PC71BM blends (the highest RRMS). The poor film morphology and quality of compound 2/PC71BM blends could increase the rate of various recombination and trapping processes during solar cell operation, leading to a low Jsc.

Figure 4.Tapping-mode AFM height images of Compound 1/PC71BM (A), Compound 2/PC71BM (B), and Compound 3/PC71BM (C) blend films from optimized solar cells. Scan size: 2 μM × 2 μM.
Fill factors (FFs) of optimized devices range from 0.44–0.49, which are typical values for solar cells made from solution-processed conjugated small molecules and smaller than typical values of polymeric solar cells (>0.6). Overall, solar cells made from current mono-DPPs have lower FFs and Jscs compared to other optimal devices made from conjugated polymers;5 although, it is possible to improve the FF of solutionprocessed small molecule solar cells using metal oxide to replace PEDOT:PSS layer.9 A potentially useful strategy to improve the PCE of DPP-containing conjugated small molecule solar cells is to increase the size of the conjugated molecules, which can be achieved by making bis- DPP compounds as displayed in Figure 1. Conjugated building blocks widely used in conjugated polymers, such as dithieno[3,2-b;2′3′-d]silole (SDT), benzo[1,2-b:4,5-b′]dithiophene (BDT), and benzothiadiazole (BT) can be introduced into these bis-DPP compounds. Because they allow for the incorporation of these additional chemical building blocks, bis-DPP compounds are capable of achieving a wider range of structures compared to mono-DPP compounds. Systematic studies of bis-DPP compounds applied as donor materials in organic solar cells are ongoing in our group and will be published elsewhere.
Field Effect Transistors
Conjugated small molecules can also be utilized as the charge transporting layer in FETs. Their p-type or n-type charge transport capabilities can be tuned by modifying their chemical structures and energy levels. Most DPP containing compounds we have investigated have p-type transport characteristics.23 However, by incorporating electron deficient groups, n-type or ambipolar transport can be achieved. Figure 5A shows the chemical structure of a bis-DPP compound (compound 4), which has a BT unit as the central unit flanked with two DPP-T3C6 building blocks. Due to the electron deficiency of the BT unit, compound 4 has lower energy levels and is capable of ambipolar transport characteristics.24 A bottom-gate, topelectrode device structure (Figure 5B) was used to measure the ambipolar transport of compound 4. Figures 3C and 3D show the typical transfer characteristics at different annealing temperatures and the corresponding carrier mobilities. Balanced carrier mobilities up to 10-3 cm2V-1s-1 were demonstrated using gold electrodes. The electron and hole mobilities can be further improved up to 10-2 cm2V-1s-1 by using a low work function electrode such as barium.
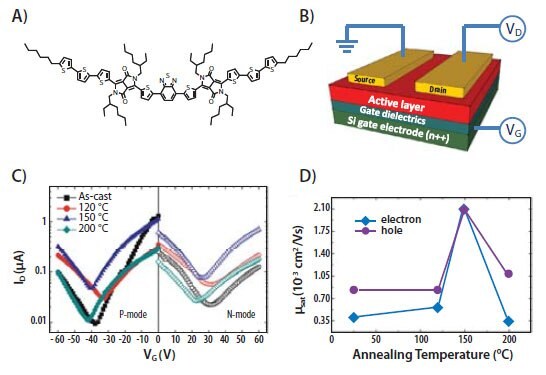
Figure 5.A) Chemical structure of compound 4, capable of ambipolar charge transport. B) A schematic illustration of FET device structure. C) Saturation transfer characteristics of FETs made from compound 4 at different annealing temperatures using Au top contacts. D) Measured carrier mobilities as a function of annealing temperature.24
Conclusions
Like their polymeric counterparts, conjugated small molecules have the potential to enable the development of inexpensive, lightweight optoelectronic devices. We briefly summarized several conjugated small molecules containing the DPP chromophore synthesized in our lab for applications in organic solar cells and field effect transistors. Conjugated small molecules allow relatively precise control over material properties such as thermal property, solubility, molecular packing, optical absorption, HOMO and LUMO energy levels, film morphology, and charge transport. This level of materials properties control results from the rational assembly of numerous chemical building blocks such as central chromophores, linkage groups, heteroatom, endcapping groups, and side chains. Using a combination of building block approach and straightforward synthesis, these materials have a vast potential application in optoelectronic devices.
Acknowledgments
We thank ONR and NSF for the financial support and all our collaborators for the contributions to this work.
Materials
References
To continue reading please sign in or create an account.
Don't Have An Account?