Recent Advances in Perovskite Solar Cells
Section Overview
- Introduction
- Early Reports on Perovskites Solar Cells (PSC)
- Materials and Preparation
- Device Configurations
- Four Main Solar Cell Design Layouts
- Mechanistic Studies
- Charge Carrier Dynamics
- Hysteresis in the I-V Curves
- Optimization of Individual Components of PSC
- Tandem Devices Based on Perovskite Solar Cells
- Large Area Modules of PSCs
- Concluding Remarks
- Related Materials
Introduction
For several decades, the need for an environmentally sustainable and commercially viable source of energy has driven extensive research aimed at achieving high-efficiency power generation systems that can be manufactured at low cost. Photovoltaic solar cells allow for the direct conversion of solar radiation to electricity using light absorbers, and recent advances in solar conversion technology have convinced many researchers that it may finally be delivering on its promise. Solar cells can be divided into three main categories based on the morphology of the light absorbers and on the device configuration.1 First- and secondgeneration solar cell devices include wafer-based silicon devices and thin film solar cells made from cadmium telluride (CdTe) or copperindium- gallium-selenide (CIGS). The technology used in these devices is considered mature and many large area modules and panels are already commercially available. These systems deliver at least 15–18% solar power conversion efficiency (PCE) with a guaranteed performance lifetime of at least 20 years. Third-generation thin film solar cells use recent advances in nanoscience and technology in order to fabricate more efficient materials and devices using low-cost solution processing methods at ambient conditions.
Devices using wide bandgap semiconductors sensitized with organic or inorganic dye molecules have attracted considerable research interest over the past two decades.2 However, the use of monolayer films of adsorbed dyes resulted in limited light absorption and caused the PCE of dye-sensitized solar cells (DSSC) to be very poor (<1%). Sol-gel chemistry permits the preparation of mesoporous oxide semiconductor thin films with controllable optical and electronic properties using synthesis of the targeted colloidal semiconductor. In 1991, O’Regan and Gratzel3 proposed use of the mesoporous semiconducting form of titania (TiO2) as a high surface area scaffold to deposit the dye in order to improve the PCE of dye-sensitized solar cells (DSSC). This new concept generated a surge in the development of DSSCs. Two device configurations of DSSCs have been studied and optimized: liquid electrolyte DSSCs where a redox electrolyte such as (I-/I3 -) present in an organic solvent functions as an electron-transfer shuttle between the counter-electrode and the photoanode, and a solid-state version of DSSC (ss-DSSC) where an organic hole transport material (HTM) is incorporated into the mesoporous oxide layer carrying the semiconductor and plays the role of the redox shuttle. To date, the highest reported solar-to-electric PCE is approximately 14.3%;4 a number of DSSC-based commercial products are already available.
Early Reports on Perovskites Solar Cells (PSC)
Perovskites are a group of structurally related materials with the generic formula ABX3 (Figure 1) where A, B, and X stand for an organic cation, metal cation, and a halide anion, respectively. For example, A = Cs+, CH3NH3 + (MA), or NH=CHNH2 + (FA); B = Pb or Sn; and X = Br, I. The perovskite family of oxides such as CaTiO3 has been known since 1893.5a In 1957, Christian Moller investigated the photoconductivity of alkalimetal mixed halides such as CsPbX3 (X= Cl, Br or I) and noted their semiconducting behavior.5b Later, Weber reported that organic analogs of ABX3, where A is an alklyammonium cation and B = Sn(II) or Pb(II) also form perovskites that exhibit interesting optical and electronic properties.5c David Mitzi provided a comprehensive review of this topic.5d By varying the alkyl chain length of the organic cation, it is possible to derive a series of organo-lead perovskites with highly tunable properties, including the optical absorption bandgap.
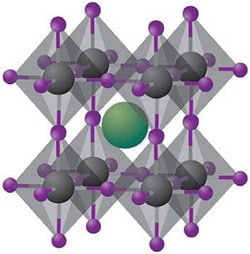
Figure 1.Cuboid structure of the hybrid halide perovskites of the type ABX3. The organic or inorganic cations occupy position A (green), whereas metal cations and halides occupy the B (grey) and X (purple) positions, respectively.
In 2009, Kojima and Miyasaka6 suggested the use of the perovskite methylammonium-lead triiodide, CH3NH3PbI3 (MAPI, Prod. No. 793833), as a photosensitizer for liquid electrolyte-based DSSCs. Lead-halide perovskites were prepared in situ by mixing the precursors lead halide and alkyl ammonium salts in an organic electrolyte, resulting in a low PCE of 3.8%. By varying the electrolyte composition and method of deposition, Park et al.7 reported improved device stability and a PCE of 6.5%, further indicating the potential of halide-perovskite photosensitizers.
Within a year of the initial advances in performance and stability of liquid-electrolyte perovskite DSSCs, a solid-state version of perovskite solar cells was reported. This new configuration used CsSnIF8 and 2,2,7,7-tetrakis(N,N-di-p-methoxyphenylamine)-9,9-spirobifluorene9 (spiro- OMeTAD, Prod. No. 792071) as HTM and resulted in an increase in PCE to 9.7% with further substantial improvement in the PSC stability. Based on these results, many laboratories across the globe took keen interest in further studying this configuration, and within a span of six years the PCE of perovskite-based solar cells (PSCs) with a typical layout of FTO/c- TiO2/meso-TiO2/perovskite/HTM/CE rapidly increased from roughly 4% to 21%.10 In this short review we shall attempt to capture the key points in the remarkable improvement in the performance of PSCs. Discussions here will focus on the research work carried out in our own laboratories in Lausanne. For a more in-depth discussion, we recommend a number of recent reviews of PSC development.11
Materials and Preparation
Three halide perovskites and their variants have been found to be efficient light absorbers: MAPI, formamidinium iodide (FAI) and the mixed halides (FA,MA)PbI3 (FAMAPI). Of these, MAPI shows a bandgap of 1.55–1.59 eV and FAI shows a bandgap of 1.45 eV. Organic-inorganic halide perovskites have several attractive features for use as light absorbers in solar cells: e.g., strong light absorption, long charge carrier lifetime (exceeding 300 ns), diffusion lengths exceeding 1 μm for electrons and holes, photoluminescence quantum efficiency as high as 70%, and ambipolar charge transport capability. Unless stated otherwise, the perovskite discussed in this paper refers to MAPI.
A very appealing feature of organic halide perovskites is their facile in situ synthesis directly on a substrate. The hybrid perovskite forms readily upon the mixing of the precursors lead halide and the alkylammonium halide in a suitable organic solvent such as dimethylformamide (DMF) under mild warm conditions. As in any sol-gel nanoparticle synthesis, considerable tunability of the electronic and morphological properties of the perovskite can be obtained by varying the synthetic conditions: e.g., the nature of the solvent, concentration of the lead salt and mixing ratio of the precursors, presence of other additives, deposition temperature, and post-annealing steps.
Three different experimental protocols have been used to prepare the perovskite layer on the substrate (as planar layers or on mesoporous oxide layers used as scaffolds): a) single step deposition using premixed solutions of the reactants MAI and PbI2 in solvents such as γ-butyrolactone, DMF or DMSO;9 b) sequential or 2-step deposition of PbX2 by spin coating followed by immersion in the solution of MAI in suitable organic solvents;12 and c) vapor phase deposition of the reactants.13 A hybrid approach combining the solution-processing and vacuum-based methods has also been used to obtain thermodynamically stable compact films with well-defined grains. Each method has its own merits, yielding PSC with high PCE >15%. MAPI is formed readily in a few seconds and can fully infiltrate the mesoporous oxide scaffolds. Near quantitative absorption of the visible light can be achieved with ca. 400– 600 nm thick perovskite layers. Regardless of which approach is ultimately used, the strong potential for cost-effective scale and manufacture of PSC technology is one of many highly appealing aspects of PSC technology.
Device Configurations
To date, the highest performing perovskite solar cells now use a high surface area mesoporous TiO2 layer as a scaffold to enhance the deposition of the perovskite light absorber. In view of the very strong absorption coefficient of hybrid halide perovskites in the visible region, the oxide layer can be substantially thinner than that used for liquid electrolyte DSSCs (150–400 nm vs. ≈10 μm in liquid-electrolyte DSSC and ≈2 μm in ss-DSSC). Mesoporous TiO2 serves as a large area scaffold for the uniform infiltration of the perovskite and also as an electron-transport layer (ETL) that collects and passes photogenerated electrons to the fluorine-doped tin oxide (FTO) collector electrode. PSC with the layout FTO/c-TiO2/meso-TiO2-MAPI/MAPI/spiro-OMeTAD/Au can be considered an n-i-p photovoltaic device with an operating mechanism similar to ss-DSSC. Upon light absorption by the halide perovskite, electrons are injected in the mesoporous layer where they percolate and eventually reach the collector electrode. The holes (h+) hop through the holeconducting material layer to reach the Au or Ag cathode.
An important property of hybrid halide perovskites is the ambipolar nature of charge carrier transport. Here, both electrons and holes exhibit very good mobility, and efficient PSCs can be fabricated even without the use of an HTM. Two experimental results support this concept. Etgar et al.14 found that solar cells made solely with the meso-TiO2/MAPI heterojunction (without any HTM layer deposition) also show a moderate PCE of 5.5%. Snaith and co-workers15 showed that a PSC made with the wider bandgap mesoporous oxide Al2O3 can achieve a satisfactory PCE as well. Both results can be rationalized only in a model where the photogenerated holes and electrons migrate efficiently to the collector (Au, TiO2) electrode, even without passing through the hole or electrontransport layer such as spiro-OMeTAD and TiO2.
In an important study, Liu and Kelly16 found that a thin layer of ZnO under a planar halide perovskite layer is sufficient to construct a high performing solar cell using the configuration FTO/ZnO ETL/planar MAPI/ spiro-OMeTAD/Au. Even in the absence of a mesoscopic oxide scaffold to aid uniform distribution of the perovskite absorber, the PSC showed a PCE of 15.7% under standard AM1.5 light conditions. Several studies17 have established that halide perovskites exhibit unusually long diffusion lengths, reaching over 100 nm for MAPI, about 1,000 nm for “MAPI3-xClx” absorber layers, and even up to 175 μm for single crystals of MAPI. The long charge carrier diffusion length renders perovskites effective as planar absorber layers. However, somewhat disconcertingly, ZnO layers have also been associated with thermal decomposition of perovskites. For example, while perovskite films deposited onto ZnO appear to be stable up to temperatures of 120 °C for short periods with little change in the absorption spectrum, the film decomposes into a yellow-colored byproduct upon heating at 100 °C for a prolonged period or even for short periods when heated to 150 °C. The absorption spectrum of these decomposed films is consistent with that of PbI2.
Snaith et al.18 first reported on an “inverted PSC design” (p-i-n architecture) using a number of organic HTM (PEDOT:PSS, V2O5 and NiO) in a solar cell with the layout FTO/PEDOT:PSS/MAPI3-xClx/PCBM/TiOx/Al. While in this configuration, holes are collected at the front transparent glass electrode via the HTM layer, electrons are collected via the ETL at the top counter electrode. In the p-i-n type devices, photocurrent flows in the reverse direction (photoinduced holes instead of electrons collected through the front conductive glass substrate) and hence the name “inverted structure.” In the initial work, the PSC with PEDOT:PSS showed a PCE of 10% on glass substrates and 6% on flexible polymer substrates. Since the initial studies, the PCE has been improved to 18%.17d
Four Main Solar Cell Design Layouts
During the past three years there have been several hundred reports of solar cells fabricated using halide perovskites. Based on the components and their layout, PSCs can be broadly divided into the following four categories: a) those based on mesoporous TiO2 or similar scaffolds (labeled mesoporous-active); b) those based on mesoporous Al2O3 scaffolds or similar oxides (labeled as mesoporous-passive or mesoporoussuperstructure); c) those based on planar absorber layers with electrontransport layer deposited on the transparent FTO/ITO (n-i-p type, labeled as planar-regular); and d) those based on an inverted layout where the hole-transport layer is deposited on FTO/ITO anode (labeled as planarinverted). Typical device layout of these four categories are shown in Figure 2.
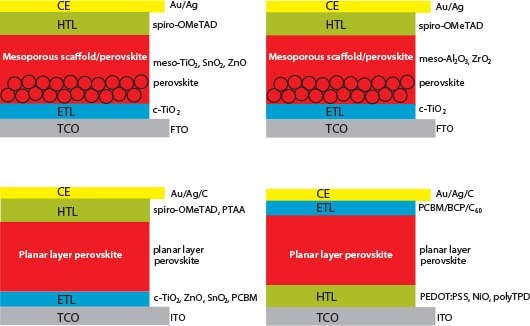
Figure 2.Schematic representation of the four main configurations of perovskite solar cells. A) Mesoporous-active: FTO/c- TiO2/mesoTiO2/perovskite/HTL/CE (Au or Ag). B) Mesoporouspassive: FTO/c-TiO2/meso Al2O3/perovskite/HTL/CE (Au or Ag). C) Planar-regular: ITO/c-TiO2(ETL)/ planar perovskite/HTL/CE (Au or Ag). D) planar-inverted: ITO/HTL (PEDOT:PSS)/planar perovskite/ ETL (PCBM-C60)/Ag.
PSC devices based on mesoporous oxide scaffolds and compact-TiO2 layers require high temperature processing, typically >400 °C. This, in turn, requires the use of rather expensive FTO in the transparent electrode. Hence, there has been a sustained search for designs that use inexpensive oxides such as ZnO to allow processing at lower temperatures (≤150 °C) or those that use low-temperature, processable organic electron- and hole-transport materials. Solar cells with planar perovskite layers (planarregular and planar-inverted) use low cost indium-doped tin oxide (ITO) electrodes. Tables 1 and 2 show data from the best performing perovskite solar cells that use mesoscopic active and passive scaffolds with PCE >15% reported in the past two years.19–22 The highest PCE (20.8%) was obtained using a mesoporous TiO2-based PSC based on a mixed cation mixed-halide perovskite film prepared in a single step from a solution of FAI, PbI2, MABr, and PbBr2 in a mixed solvent containing dimethyl formamide (DMF) and dimethyl sulfoxide (DMSO). To date, PSCs with the structure (Au/spiro-OMeTAD/perovskite/meso-TiO2/c-TiO2/FTO) deliver the highest photocurrent and photovoltage, also with minimal hysteresis.
Mechanistic Studies
In addition to extensive performance evaluations, the optical, electronic, and photoluminescence properties of perovskite sensitizers have been extensively studied and revealed several attractive features of this family of light absorbers. The excitons produced by light absorption have a weak binding energy of about 30 mV, indicating that most of them dissociate very rapidly as free carriers at room temperature. Solar cells based on such sensitizers have the potential to deliver photovoltage close to the bandgap energy. The resulting electrons and holes exhibit a small effective mass, yielding high carrier mobilities ranging from 7.5 cm2V–1s–1 for electrons to 12.5 cm2V–1s–1 for the holes. Snaith et al.17d measured effective mobilities of 11.6 cm2V–1s–1 for MAPI3-xClx and ~8 cm2V–1s–1 for MAPI, exceptionally high for solution-processed materials. This surpasses charge mobilities reported for the mesoporous TiO<sub>2</sub> used in DSSC by at least a factor of 20, and those of typical π-conjugated molecular semiconductors by several orders of magnitude. The recombination of the charge carriers occurs relatively slowly at hundreds of nanoseconds and results in long carrier diffusion lengths (100 nm to 1,000 nm).17 The long diffusion length implies that charges can be transported over much longer distances than the absorption length of light in the material, rendering highly efficient collection.
Conceptually, the operational principle of PSCs is similar to that of both dye-sensitized and inorganic solar cells. Optical excitation within the perovskite film is followed by electron transfer to the TiO2 scaffold and/ or hole transfer to the HTM. These processes must be kinetically fast in comparison to the recombination rates of the photogenerated species and also compared to the rate of back electron transfer at the interfaces between the TiO2/HTM/perovskite materials. For mesoscopic systems, interfacial properties largely determine the device performance. Halide perovskites have small exciton binding energy (≤50 mV) and in planar devices photogenerated bound excitons readily dissociate (in few ps) to free charge carriers. The free electrons and holes both have high mobilities (≈10–30 cm2V–1s–1) to diffuse apart. Optimal separation and collection of electrons and holes at the collector electrode requires their diffusion lengths to be several times longer than the film thickness required for quantitative light absorption. The high efficiency observed in several planar devices indicates that for these processes a typical absorber layer thickness of 400 nm is required.
Charge Carrier Dynamics
A comparative study of charge transport processes in the three different device architectures (mesoporous TiO2 scaffold, mesoporous Al2O3, and planar) was made using visible pump, visible-NIR probe transient absorption spectroscopy.23 In PSCs composed of mesoporous Al2O3, electron injection from the excited perovskite to the oxide is blocked by virtue of the high-energy conduction band level. In this case, the interfacial charge separation is effected by hole transfer to the HTM; injected electrons are transported through the perovskite absorber. In the planar layer case, no electron injection takes place because the photogenerated charge carriers migrate through the perovskite layer to reach the electron and hole-selective interfaces and get separated across these heterojunctions. In all three cases, hole transfer to the HTM takes place. When exposed to ambient conditions, coated mesoporous-Al2O3 and planar systems show a rapid and significant degradation in the yield of long-lived charge separation. This process occurs when both light and oxygen are present but does not affect the mesoporous TiO2 scaffold case.
Hysteresis in the I-V Curves
A lingering issue with PSCs is the observation of a scan-rate dependent hysteresis phenomenon in the current-voltage curves.24 Specifically, photocurrent variation with voltage in the forward and reverse sweep directions is not the same. The magnitude of hysteresis depends on the sweep onset voltage and the direction (from positive to negative values during backward scan or in the opposite forward scan). The hysteresis is observed to varying degrees in all three device architectures and regardless of the morphology of the perovskite layer. It is not caused by the presence of a compact TiO2 layer or due to the hole transport material used and appears to be inherent to the perovskites and associated interfaces. This hysteresis renders standard PCE measurements ambiguous (overall PCE is higher with faster scan rates). Several explanations have been proposed for this phenomenon: very slow trapping and de-trapping of charges, ferroelectric behavior of the perovskites on nanosecond timescale, slow ion migration or displacement, and chemical structural changes. Accurate measurements of PCE must, therefore, be made using extremely slow scan rates or by measuring the stabilized power output at maximum power-point and by tracking the power output until it reaches a constant value. Additionally, few hysteresis-free PSC devices with inverted structure (FTO/PEDOT:PSS/MAPI/PCBM/BCP/Ag) having a PCE of 18.1% have been reported.24f
Optimization of Individual Components of PSC
Here we will review some of the optimization studies where alternatives have been tested by replacing one or more of the key components of the perovskite. These include the oxide layer, the metal ion, the halide and organic cation of the perovskite, the electron-transport and hole transport layers, and the counter electrode.
Alkyl Ammonium Halides
A majority of perovskite solar cell studies utilize the hybrid perovskite MAPI. Several studies have also used the mixed halide (labeled as CH3NH3I3-xClx) that forms when PbCl2 is used as a reactant for the methylammonium halide. This mixed halide has a bandgap comparable to the MAPI, but it exhibits an order of magnitude longer carrier diffusion length and performs equally well.
The tribromide analog CH3NH3PbBr3 (MAPBr) has a higher bandgap (2.3 eV) compared to MAPI. Hence, any device based on MAPBr is expected to deliver a lower PCE. This expectation has, indeed, been confirmed in a passive PSC with mesoporous alumina Al2O3 scaffold. A graded series of mixed halide perovskites were prepared by mixing different ratios of MAI and MABr to yield perovskites with bandgaps between 1.55 and 2.3 eV. The mixed perovskite MAPI2Br yields a 200 mV higher bandgap than MAPI (1.78 eV). Ultraviolet photoelectron spectroscopy studies indicate that the valance band edge of MAPI3 and MAPI2Br are similar (–5.40 eV) but their conduction band edges are different (–3.86 eV for MAPI3 vs. –3.62 eV for MAPI2Br). As expected for a solar cell device based on TiO2 nanorods, the PCE for the devices increases from 4.29% for MAPI3 to 4.89% for the MAPI2Br.25
Similarly, the mixed-organic-cation perovskite MA0.6FA0.4PbI3 performed better (14.9% PCE) than both unmixed PSCs.19m The methylammonium cation can be replaced by the larger formamidinium cation, [NH2CH=NH2+ (FA), and the resulting formamidinium triiodide (FAPI) shows a smaller bandgap (1.48 eV FAPI vs. 1.55 eV for MAPI)].26 Solar cell devices prepared using FAPI exhibit strong photoluminescence, long lifetimes, higher PCE, and minimal hysteresis. In a recent study, a mixed halide perovskite film was prepared in a one-step procedure from a mixture of FAI, MABr, PbI2 and PbBr2.19a A solar cell device with mesoporous TiO2 and spiro-OMeTAD as the hole transport layer showed a record high PCE of 20.8% for a PbI2/FAI molar ratio of 1.05 in the precursor solution. The photovoltage obtained (1.18 V) was also the highest value reported for any PSC to date.
Other Metal Ion Halide Perovskites
To avoid the use of toxic lead salts, researchers focused on replacing lead with two members of the same group, Sn and Ge. One problem in the preparation of Sn(II) derivatives is the instability of the resulting devices in the presence of oxygen and moisture. Using encapsulation under inert atmosphere, stable Sn(II) halide perovskite CH3NH3SnI3 has been prepared and tested.27 This Sn(II) perovskite has a bandgap of 1.3 eV, with charge carrier mobility of ≈1.6 cm2V–1s–1. It exhibits diffusion length of ca. 30 nm, shorter than the more than 1 μm observed in the corresponding Pb(II) perovskite. The best performing Sn-based device (FTO/c-TiO2/meso-TiO2/ MASnI3/spiro-OMeTAD/Ag) reaches a power efficiency of 6.4% under 1-sun illumination. Quite remarkably for an absorber with a bandgap of 1.23 eV, the open circuit voltage is as high as 0.88 V in the most efficient device.
Kanatzidis et al.27 explored bandgap engineering of Sn(II) perovskites using chemical substitution in the form of CH3NH3SnI3-xBrx solid solutions. Among MASnI3, MASnI2Br, MASnIBr2, and MASnBr3 salts, solar cells with the dibromide derivate MASnIBr2 (with a bandgap of 1.75 eV) show the highest PCE of 5.73%.
Optimization of the Oxide Anode/ETL
Similar to conventional DSSCs, there have been many optimization studies where different morphological forms—one-dimensional (1D), and three-dimensional (3D) and hierarchical structures—and doping of the semiconducting oxide layer(s) were used in PSCs. The compact layer of TiO2 serves as an ETL and as a block for the perovskite absorber and the hole transporter that comes into direct contact with the transparent conducting oxide anode. Presence of the c-TiO2 layer is, thus, essential in all three (passive, active, and planar) PSC devices.
Because the 3D nature of the mesoporous titania layer is widely believed to reduce electron mobility, a number of different forms of TiO2 have been examined, including 1D nanostructures such as nanosheets, nanowires/fibers, nanorods, and nanotubes.28 One such study reports a PCE of 14.8% for a PSC made of highly transparent TiO2 anatase nanotube arrays. PSCs made of rutile nanorods show a PCE of 9.4%. Onedimensional nanostructures present a fairly low surface area compared to their mesoporous counterparts. Hierarchical structures grown on 1D nanostructures provide higher surface area and have seen a 25% increase in the photocurrents with constant photovoltages. Mg or Y-doping of TiO2 gives rise to an increased PCE, presumably due to the widening of the TiO2 bandgap by 100 mV and related better light harvesting by the perovskite.
SnO2 has several attractive properties relative to TiO2. It exhibits a higher bandgap (3.8 eV), reducing any photocatalytic activity caused by near UV component of solar radiation and rendering higher device stability. It also exhibits charge carrier mobility up to 240 cm2V–1s–1 (two orders of magnitude higher) with low density of trap states, and it possesses a deeper conduction band that facilitates a more efficient transfer of photogenerated electrons. There have been few studies of PSC using SnO2 anodes.29 A record solar conversion efficiency of 18.4% was obtained22b for a PSC based on SnO2 as ETL and a mixed halide perovskite MAPBr3(15%)- FAPI3 (85%).
ZnO has a bandgap similar to that of TiO2 (3.3 eV) with comparable electron affinity. However the conductivity of ZnO is several orders of magnitude higher than that of TiO2, making it an attractive ETL. As in the case of nanorods of TiO2, PSCs based on ZnO nanorods achieve comparatively inferior performance (PCE 11%). Liu and Kelly21h obtained a PCE of 15.7% on a PSC based on a compact ZnO as ETL over which a planar layer of MAPI3-xClx was used as the light absorber. A PSC based on sputtered compact films of ZnO and MAPI30 (ITO/ZnO/MAPI/spiro- OMeTAD/Ag) shows similar PCE values (15.9%). For passive forms of mesoscopic oxides, ZrO2 was found31 to be equally effective as alumina, and PSCs made using this configuration exhibit comparable PCE (10.8% with ZrO2 vs. 10.9% with mesoporous Al2O3).
Back Contact
Generally, a thin film of noble metal (Au or Ag) is deposited on top of the hole transport layer to serve as the back contact in solar cells. In a costsaving approach, attempts have been made to use various allotropes of carbon as a counter electrode. Mhaisalkar et al.32 managed to successfully transfer carbon nanotubes onto the HTM in order to construct a PSC with mesoporous TiO2 as an “active” scaffold and spiro-OMeTAD as the HTM. The MAPI/CNTs solar cell showed an efficiency of up to 6.87%, demonstrating the potential of applying carbon nanotubes as a charge collector, eliminating both the metal electrode and hole transporter in perovskite solar cells. The addition of the hole transporter spiro-OMeTAD over the perovskite improved the efficiency to 9.90%.
Graphite–carbon black paste has also been used to prepare a mesoporous carbon counter electrode and use it to construct an HTMfree PSC device.33 A PSC based on mesoporous layers of ZrO2 and Carbon (FTO/c-TiO2/meso-TiO2/MAPI/ZrO2/C) displays a remarkable PCE of 11.63%. Compared with Au or Pt CEs, printable carbon counter electrodes are much cheaper and easier to process, especially for large-scale production.
Hole Transport Materials
Prompt and quantitative capture of photogenerated charge carriers is essential to deliver maximum PCE. Hence, there has been a great effort to identify newer, more efficient electron transport materials (ETM) and HTM. For the best performance, hole transport mediators must meet several requirements. Along with the high hole mobility and good solubility in organic solvents, it must have good compatibility between its HOMO energy levels and the valence band level of the perovskite. High hole mobility permits fast extraction of charges and a higher photocurrent. Sluggish movement of holes leads to higher internal resistance and this in turn reduces the fill factor of devices. Based on the chemical composition, HTM can be grouped into three categories: inorganic, small molecule (organic), and polymeric.
The best HTM used in PSCs has been spiro-OMeTAD (Figure 3), which belongs to the small molecule HTM group. A PCE of >18% has been obtained by applying this material. In spite of its excellent performance, this compound has some limitations. The oxidized form of the spiro- OMeTAD exhibits absorption in the visible light region with a maximum at 520 nm, essentially acting as a filter. The conductivity and hole mobility of pristine spiro-OMeTAD are quite low (8.7 × 10–5 Scm–1 and 4 × 10–5 cm2V–1s–1). These values increase by an order of magnitude upon addition of select p-dopants, although it would be ideal to have a HTM that functions well without any further dopants.
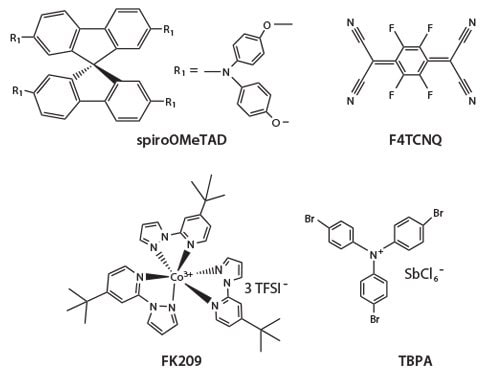
Figure 3.Chemical structure spiro-OMeTAD and some of its p-dopants.
Several additives, mostly metal complexes or salts have been tested as dopants: (p-BrC6H4)3NSbCl6, Co(III) complexes such as tris(2-(1H-pyrazol- 1-yl)pyridine)cobalt(III) (FK102), lithium bis(trifluoromethanesulfonyl) imide (LiTFSI), SnCl4, SbCl5 and FeCl3, WO3, molybdenum tris[1,2- bis(trifluoromethyl)ethane-1, 2-dithiolene], and F4-TCNQ. Most of them are usually applied by vacuum deposition in order to improve electrical conductivity, electron injection, and to reduce recombination. Li ions tend to insert onto the TiO2 film, altering its electronic properties. The cobalt(III) complex FK209 shows much better solubility in the spiro-OMeTAD-based precursor solution.
Specific examples of HTM that have been found effective with PCE ≥7% include CuSCN, CuI, NiO, V2O5, and graphene oxide (GO)—typical inorganic HTMs. Typical examples of small molecule HTMs are spiro- OMeTAD and its extended derivatives; carbazole-based V886, X19, X51, and SGT 405; spiro-acridine-fluorene based CW4; silalothiophenebased triphenylamines; quinolizino acridines; tetra-azulene derivatives, thiophene-based H101 and H111; tetraphenylbenzidine-TPB-based TPBC; and triptycene derivative T103. The donor-acceptor properties of several organic aromatic molecules with a delocalized π-system have been adjusted by the introduction of various functional units to form the second category of small-molecule HTMs. Examples of this type of HTM include functionalized derivatives of pyrene, thiophene and bithiophenes, perylene, carbazoles, triarylamines, tetrathiafulvalene, spirofluorenelinked triphenylamines such as spiro-OMeTAD, and TPB. The third group, polymeric HTMs, are efficient in bulk heterojunction polymer organic solar cells. Typical examples of polymeric HTMs are thiophene-based P3HT, triarylamine-based PTAA, phenyl benzidine-based polyTPD, thiophenebased PCPDTBT, PCDTBT, and PCBTDPP and polyfluorenes such as TFO, PFB, and TEB (Figure 4).
Table 3 presents data on some of the best-performing HTMs examined in PSCs having the mesoscopic active scaffold of TiO2.34 All of these studies employ either MAPI or its chloride analog MAPI3-xClx. The bandgap energy of these two materials is very similar (1.55–1.58 eV). With the same choice of electron transport layer (TiO2), it is meaningful to compare the PCE obtained with different HTMs. With a judiciously tailored design of an HTM, researchers have attained PCE values greater than 18%. A unique, distinguishing feature of PSCs is high photovoltage (≥1 V) obtainable with light absorbers of bandgap of 1.5–1.6 eV. Intrinsic losses attributable to the direct bandgap perovskite light absorber are low due to low exciton binding energy (≤50 meV), long electron/hole diffusion length (≥100 nm), and high mobility of holes and electrons (in the range 1–10 cm2V–1s–1). The ratio of Voc/Ebg is ≈0.69, which is higher than that of polymer organic solar cells (≈0.55). However, this value is still lower than the 0.80 obtained for thin film amorphous-Si and GaAs thin film solar cells. Proper alignment of the energy levels of the ETL and HTM with respect to the valence and conduction band energy levels of the perovskite is essential to improve the light harvesting efficiency. Examination of the data collected in Table 3 shows a decreasing PCE value is accompanied by a systematic decrease in the Voc and fill factor of the solar cell device. A higher fill factor is obtainable in devices with a sheet resistance and a high shunt resistance.
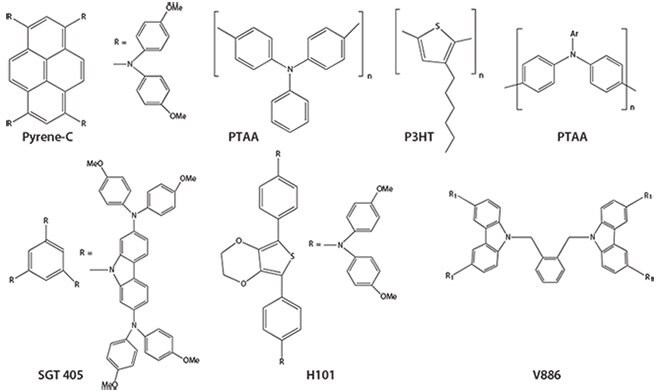
Figure 4.Some examples of polymeric hole transport materials.
Tandem Devices Based on Perovskite Solar Cells
Halide PSCs made with MAPI absorb light efficiently in the blue-green region of the solar spectrum. These PSCs show a clear transparent window at longer wavelengths (>800 nm), opening up the possible design of tandem solar cell packs. A low bandgap solar cell made of Si or CIGS placed as the bottom cell can collect all the light in the red and near-IR region. A tandem device made up of amorphous Si (Ag/AZO/a-Si:H/ c-Si/a-Si:H(ITO) has been successfully connected to a top PSC using a thin layer of transparent SnO2 deposited on the top of the Si solar cell.35a The tandem device of the (Ag/a-Si:H/c-Si/aSi:H/ITO/SnO2/perovskite/ spiroOMeTAD/MoO3/ITO) layout showed an efficiency of 18%, the highest value for this type of cell architecture. Thin MoO3 serves as a protective layer between this hole conductor and the transparent top electrode of ITO. The back side of the Si cell is fully covered with aluminum-doped zinc oxide (AZO) and silver, both deposited by sputtering. Simulation studies35b of a tandem device made up of MAPI PSC and Si solar cell indicate that with optimized layer thickness the tandem device can deliver efficiency of >30%. A study of a tandem device composed of CIGS as the bottom cell and a perovskite on the top has also been made.35c
Large Area Modules of PSCs
During the past year there have been few successful reports on the fabrication of large area PSC modules. Razza et al.36a optimized the sequential deposition method of halide perovskites to prepare 10 mm2 solar cells that achieved a maximum efficiency of 13.3% and an average efficiency of 12.1%. To prove the scalability of the process, seriesconnected modules were fabricated containing blade-coated PbI2 films. A module efficiency of 10.4% was then obtained for a 10.1 cm2 active area, and an efficiency of 4.3% was measured for a module area of 100 cm2.
In a related study, Yang et al.36b used a large excess of organic halide anion during the two-step perovskite preparation and anti-solvent to grow large grain-sized crystals. This achieved a PCE of 16.3% for a planar PSC with 1.2 cm2 active area (stabilized PCE output of ≈15.6%). When the device area was reduced to 0.12 cm2, a maximum PCE of 18.3% was achieved (stabilized output of ≈17.5%). In 2015, researchers at IMEC, Belgium, reported successful preparation of a perovskite solar cell with 16 cm2 active area and a PCE of 11.9%.
An important experimental strategy was recently developed36c to prepare heavily doped inorganic charge extraction layers in planar PSCs to achieve very rapid carrier extraction, avoid pinholes, and eliminate local structural defects over large areas even with 10- to 20-nanometer-thick layers. The robust inorganic nature of the layers enabled fabrication of PSCs with an aperture area >1 cm2 and a PCE >15%. Hysteresis in the current-voltage characteristics was eliminated, creating stable PSCs with >90% of the initial PCE remaining after 1,000 hours of light exposure.
Concluding Remarks
Progress in the development of high-efficiency solar cells has been so remarkable that within the last three years the solar conversion efficiency has rapidly soared to 21%. However, a number of important conditions must be achieved for hybrid halide perovskite-based solar cells to reach their commercial potential.
- First, improved understanding and control of experimental conditions is required in order to enable easy replication of results across different laboratories. For example, preparative conditions vary significantly, and these conditions strongly influence the crystalline (electronic) and morphological properties of the perovskite layer. This, in turn, drastically affects the overall PCE. Hysteresis observed in the I-V curves requires that photocurrent and voltage must be measured under very slow sweep rate conditions.
- Second, promising solar efficiencies must be replicated in large area modules and panels. Solar cells used in fundamental studies are typically small in size (≤1 cm2), and confidence in the reproducibility of a given method requires measurement in large area samples. Few encouraging reports have appeared recently on the preparation of such large area modules (≥10 cm2) with good efficiency (>10%).
- Third, long-term stability of these new solar devices must be demonstrated under realistic field operating conditions that include elevated temperatures, high moisture/humidity, and constant exposure to light. Few select design layout and material preparation methods have been identified in which the solar cell shows stable performance of at least 1,000 hours under conditions of full solar light exposure or heating to 85 °C. Extensive investigation and development will be required to fully address the moisture sensitivity of PSCs. Either a non-toxic replacement for lead-based perovskite materials and/or a highly robust encapsulation technique will be needed to prevent negative environmental impacts from this technology.
- Last, in order to unleash the enormous potential of PSCs, successful large-scale, low-cost manufacturing methods must be developed.
Related Materials
References
To continue reading please sign in or create an account.
Don't Have An Account?