Development of Small Molecule Donors for Solution-Processed Organic Solar Cells
Introduction
Solution-processed organic photovoltaic devices (OPVs) have emerged as a promising clean energy generating technology due to their ease of fabrication, potential to enable low-cost manufacturing via printing or coating techniques, and ability to be incorporated onto light weight, flexible substrates.1 The most successful class of OPV devices are those with active layers composed of blended polymeric electron donors and fullerene acceptors, widely known as polymer–fullerene bulk heterojunction (BHJ) solar cells.2 Power conversion efficiencies (PCE) for these systems have reached 9.2% for single-layer devices3 having already shown great promise as renewable, lightweight and low-cost energy sources. Recently, the power-conversion efficiency of stateof- the-art PSCs has exceeded 8% in the scientific literature. However, to find viable applications for this emerging photovoltaic technology, further enhancements in efficiency will be required to increase efficiency and achieve the required performance threshold (10% for commercial applications and 10.6% for tandem cells).4
Recently, several new classes of small molecule donors with favorable optical and electronic properties have been reported for use in OPV devices.5,6 These small molecules offer advantages over their polymeric counterparts: (a) their structures are well defined and exhibit no molecular weight dependence, leading to improved purity and limiting batch-to-batch variation; and (b) they typically exhibit more organized nanostructures, leading to higher charge carrier mobilities. Additionally, small molecule architectures are sensitive to subtle structure changes; thus, electronic energy levels, optical absorption, and self-assembly tendencies can be systemically tuned to maximize device performance. When coupled with fullerene electron acceptors such as [6,6]-phenyl- C71-butyric acid methyl ester (PC71BM) (Prod. No. 684465), small molecule-based solar cells have achieved record PCEs over 8%.7,8 This article highlights the progress of small molecule-based organic solar cells over the past few years and describes key structural features that have led to this performance improvement.
Section Overview
- Device Operation and Donor Molecular Design
- Initial Donor Small Molecules
- High-performance Donor Small Molecules
- Conclusions and Future Outlook
- Related Products
- References
Device Operation and Donor Molecular Design
While a detailed analysis of photocurrent generation in OPVs1-3 is beyond the scope of this article, it is important to recognize that organic solar cells operate on the principle that two materials are required to generate free charge carriers. A simplified view of device operation is shown in Figure 1A. Combinations of materials with high ionization potentials (electron donor) and high electron affinity (electron acceptor) are responsible for light absorption, exciton formation, charge separation, and charge transport. Ubiquitous to OPV devices is the use of soluble fullerene derivatives as the electron acceptor owing to their low lying, lowest unoccupied molecular orbital levels and isotropic electron mobility (Figure 1B).
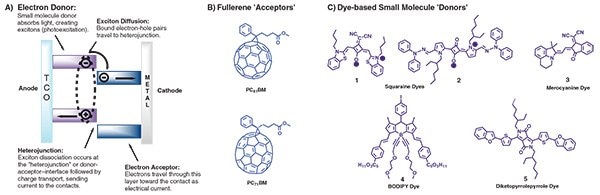
Figure 1.A) Simplified figure showing organic solar cells device operation (TCO = transparent conducting oxide). Chemical structures of B) commonly used soluble fullerene derivatives, and C) early examples of small molecule donors constructed from common organic dyes.
The vast majority of OPV performance improvements has been a result of the development of new ‘donor’ materials that are designed to maximize light absorption, transport charge, and form ordered nanostructures when blended with fullerenes. While π-conjugated polymers have received the most attention, small molecules can be strategically designed for use as effective ‘donor’ materials.
There are several key parameters to consider when designing small molecule donors to be paired with fullerene acceptors in solutionprocessed BHJ solar cells, including: (a) strong optical absorption that extends into the near-IR region (λmax should be centered around 700 nm, the region of maximum photon flux) and extinction coefficients (ε) greater than 50,000 M-1 cm-1 are desired to maximize photon harvesting; (b) relatively deep highest occupied molecular orbital (HOMO) energy levels from –5 to –5.5 eV to maximize open circuit voltages, while still matching commonly used high work function anodes; (c) relatively planar structures to promote intermolecular π–π interactions which are important for achieving high charge carrier mobility; (d) sufficient solution viscosity and solubility to enable thin film formation via solution deposition; and (e) synthetic procedures that are simple, high yielding, and highly tunable to ensure both gram quantities can be made and molecular libraries can be created. With these considerations in mind, several research groups have developed a series of solution-processable small molecules that challenge those of the very best polymers in solar cell applications.
Initial Donor Small Molecules
The development of high-performance solution-processed small molecule bulk heterojunction solar cells (SM-BHJ) did not happen overnight. Rather, there has been a long history of work in the area coming from a few research groups. Historically, small molecule donors have tended to be just that, small. They exhibited difficulties in forming uniform thin films from solution and, thus, have primarily been utilized in thermally evaporated devices.9 In the mid-2000s, Roncali et al. developed a series of tetrahedral-shaped oligothiophene molecular donors that could be solution-processed with PC61BM to produce solar cells with a PCE of ~0.2%.10 Around the same time, Anthony et al. reported a series of soluble acene derivatives which achieved PCEs of ~1% when paired with PC61BM.11 A major problem with these initial compounds was poor spectral overlap with the solar spectrum and limited optical absorption beyond 600 nm. None-the-less, these initial results demonstrated that small molecule donors could indeed be processed from solution to give working solar cell devices. Overcoming the issue of poor light harvesting properties, a series of papers was published between 2008 and 2010 describing the functionalization of strongly absorbing dyes. Zeisel and Roncali showed that highly absorbing (ε > 100,000 M-1cm‑1) BODIPY dyes (Prod. Nos. 745820, 746169, 747106, and 790389) could be made soluble and form films from solution by attaching oligooxyethylene chains to the π-conjugated backbone. When incorporated into devices with PC61BM, PCEs in excess of 1% were obtained and, most importantly, photo-current generation beyond 750 nm was achieved.12 The PCE of BODPIY-based SM-BHJ solar cells has since been improved to ~5%.13 Both Marks14 and Wurthner15 research groups reported on functionalized squaraine dyes (Prod. Nos. 757233, 757268, 757276, and 758337) that exhibited broad and intense thin film absorption spectra extending well into the near IR. Devices with PC61BM gave PCEs on the order of 1–2%. These devices ultimately suffered from small open circuit voltages (Voc) and poor fill factors, a result of high-lying HOMO levels and poor active layer morphologies.
Wurthner also successfully incorporated merocyanine dyes (Prod. No. 323756) into BHJ solar cells. Such dyes are easily synthesized and exhibited strong absorption in the red region of the solar spectrum. PCEs upward of 2.5% were obtained upon solution-processing the dye with soluble fullerene derivatives.16 These devices had a higher Voc in comparison to the squaraine-based devices, yet once more suffered from poor fill factors. In the late 2000s, Nguyen et al. utilized the strongly absorbing diketopyrrolepyrrole dye (Prod. Nos. 753912 and 753920) to construct a series of narrow band gap, solution-processable small molecules for use in OPV devices. One such derivative incorporated 2-ethylhexyl alkyl chains on the amide N-atom to enable dissolution in organic solvents and 2-benzofuran units on the terminal positions to extend π-delocalization and direct self-assembly. This molecule, named DPP(ThBzFu)2, had near ideal optical and electronic properties for use as a donor molecule in SM-BHJ solar cells, including strong optical absorption beyond 700 nm, a deep HOMO level, and a simple three-step synthesis from commercially available starting materials. Solar cells fabricated with DPP(ThBzFu)2:PC71BM active layers yielded PCEs in excess of 4%, a record that stood for two years.17 Important parameters for achieving such a high PCE were the use of high concentrations of DPP(ThBzFu)2 compared to PC71BM and thermal annealing of the active layer to achieve appropriate nanoscale phase separation, thus indicating the importance of active layer processing. Ultimately, this initial work on soluble small molecule donors by Roncali, Anthony, Marks, Wurthner, Nguyen and others set the stage for major breakthroughs in subsequent years. Three excellent reviews by Nguyen18, Baurele19, and Zhan20 provide a more detailed analysis of the field.
High-performance Donor Small Molecules
Building on the established literature, and taking into account the specific criteria to develop small molecule donors, Heeger and Bazan reported on a highly modular molecular framework found to yield materials with near ideal properties for use as donor molecules in BHJ solar cells.5,21 The architecture consisted of an acceptor-donor-acceptor (ADA) core flanked with end-capping units. Two acceptors were utilized to increase the electron affinity across the conjugated backbone ensuring deep HOMO levels, while end-capping units served to extend π-conjugation and tailor self-assembly properties. Important to this class of compounds was the use of a pyridyl[2,1,3]thiadiazole (PT) building block as the electron acceptor, which promoted strong intramolecular charge transfer when coupled with electron donors, resulting in narrow band gaps. Additionally, the asymmetrical nature imparted selective reactivity, allowing for the synthesis of monofunctionalized materials in high yield.
Two synthetic entries into this class of compounds are shown in Figure 2A. In Method 1, small molecules are built from the inside out, leading to a final architecture where the pyridyl N-atoms are in a position proximal to the donor core; in Method 2, small molecules are built from the outside in, leaving the pyridyl N-atoms in a position distal to the donor core. Through these simple three-step syntheses, a whole series of small molecules were made incorporating a range of donor cores and end capping units (Figure 2B). Among the many derivatives synthesized, it was found that utilizing a 2-ethylhexyl-substituted dithienosilole (DTS) donor and 2-hexylbithiophene end-capping units resulted in virtually ideal optical and electronic properties. The compound d-DTS(PTT2)2 (6) shown in Figure 2C exhibited strong long wavelength absorption with peak absorption at 700 nm in the solid state (near the region of max photon flux) and HOMO and LUMO levels at –5.2 eV and –3.6 eV, respectively, making it compatible with fullerene derivatives. High organic solvent solubility (>20 mg/mL), as a result of alkyl side chains both perpendicular and parallel to the molecular backbone, allows for uniform film formation. In addition, the planarity of the small molecule, important for intramolecular π-delocalization and intermolecular π-stacking, resulted in high charge carrier mobilites on the order of 0.1 cm-1 V/s. Incorporating d-DTS(PTT2)2 into BHJ devices with PC71BM produced initial PCEs of 3.2%. By optimizing device architecture and processing conditions, PCEs were improved to 5.6% through the use of molybdenum oxide (Prod. No. 203815) hole transport layers and processing with small amounts of 1,8-diiodooctane (DIO) (Prod. No. 250295). To further improve device performance, Bazan and co-workers employed the regio-isomer p-DTS(PTT2)2 [or p-DTS(PTTh2)2, Prod. No. 772372, (7)], where the pyridyl N-atoms are positioned proximal to the central DTS unit. A slight change in the molecular geometry toward a more ‘banana’ shape, resulted in a greater tendency for p-DTS(PTT2)2 to self-assemble, leading to higher hole mobilities, increased light absorption, and ultimately a higher PCE of 6.7%.21 This result represented, for the first time, a solution-processed small molecule BHJ device comparable in PCE to its polymer counterparts. Additionally, replacing the pyridyl N-atom for a C–F bond p-DTS(FBPTT2)2 [or p-DTS(FBPTTh2)2, Prod. No. 772380, (8)], improved the stability of the compound and resulted in PCEs in excess of 7% when processed under the same conditions. Further device optimization has led to PCEs approaching 9%.7 Quite interestingly, analogous molecules utilizing the ubiquitous benzo[2,1,3]thiadiazole acceptor, compound 9, exhibited no photovoltaic behavior when processed under similar conditions due to an inability of 9 to form ordered nanostructures required for rapid charge transport.5 These results highlighted how subtle alterations to molecular structure can greatly impact self-assembly processes and, in turn, device performance.
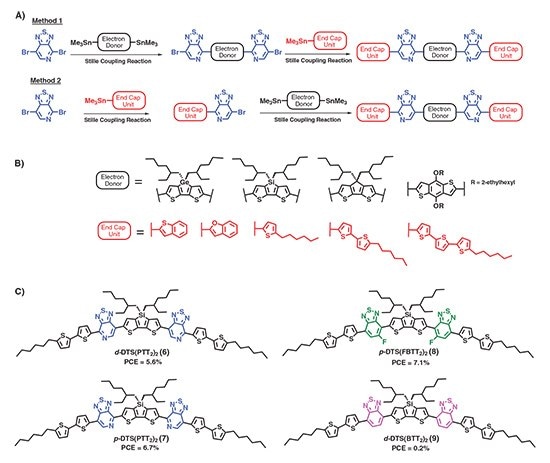
Figure 2.Chemical structures of soluble small molecule donors reported by Bazan and co-workers. A) General three-step synthetic pathway toward end-capped ADA-type molecules with proximal (Method 1) and distal (Method 2) regio-chemistry of the pyridyl N-atom. B) Examples of building blocks used as donors and end caps. C) Best performing architecture showing the influence of heteroatom substitution on power conversion efficiency.
In a parallel development, Chen et al. reported a series of narrow band gap oligothiophenes that exhibited excellent performance when incorporated into SM-BHJ solar cells.6 They demonstrated that end capping long septithiophenes with electron withdrawing units provided materials with absorption profiles extending into the red region of the solar spectrum and deep HOMO levels desired to give large Voc. Critical to these materials was the incorporation of long alkyl side chains on six of the thiophene units which rendered the compounds highly soluble in common organic solvents and enabled the formation of uniform thin films from solution. Initial derivatives with dicyanovinyl end-cap units (Figure 3A, 10) were incorporated in BHJ solar cells with PC61BM, yielding devices with PCEs of 2.5%, which were further improved to 3.7% with device optimization. Through a systematic screening of endcapping units, Chen et al. showed that absorption profiles, HOMO/LUMO levels, and material solubility could be readily tuned. Small molecules incorporating 1,3-dimethylpyrimidine-2,4,6(1H,3H,5H)-trione (11), indan(1,3)dione (12), n-octyl cyanoacetate (13), and 3-ethylrhodanine (14) as end capping units gave the best performance with PCEs ranging from 4–6%. Solar cells based on 12 and PC61BM had extremely high fill factors of 72% due to the strong tendency for the indan(1,3)dione moiety to direct self-assembly. Compound 12 exhibited a remarkably high solubility in chloroform at >200 mg/mL, which is important for forming thicker films (>300 nm) required for large-scale fabrication. Overall, compound 14 with 3-ethylrhodanine end-capping units exhibited a good combination of high organic solvent solubility and, thus, good film forming properties and long wavelength absorption, leading to the highest PCE of the series. Replacement of the central thiophene ring in 14 with the larger and more rigid benzobithiophene conjugated unit led to compounds with improved charge transport properties resulting in increased device performance (PCE=7.4% for compound 15). Increasing the π-conjugation orthogonal to the backbone improved photocurrent generation and, with the use of a polydimethylsiloxane (PDMS) (Prod. Nos. 423785, 482064, and 482145) processing additive, achieved record PCEs of 8.1%.8 These findings brought great attention to the area and helped establish solution-processed SM-BHJ solar cells as one of the most promising clean energy technologies.
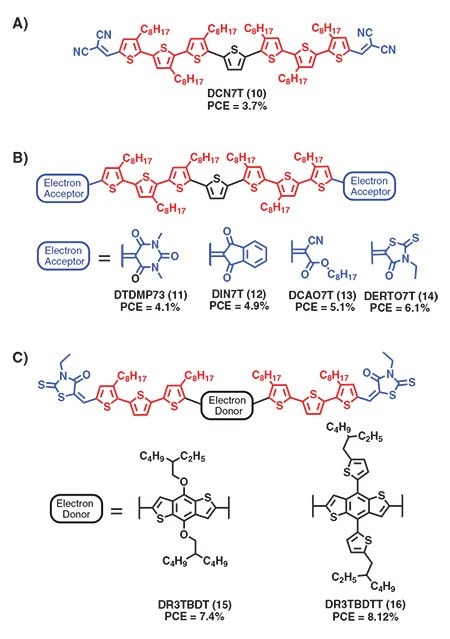
Figure 3.Chemical structures of soluble small molecule donors reported by Chen and coworkers.6 A) First generation structure consisting of a septithiophene core end-capped with dicyanovinyl units. B) Evolution of terminal end-capping units to give high performance materials. C) Small molecules with benzobithiophene based π-conjugated core units yielding best performing materials.
Conclusions and Future Outlook
Over the past four years, creative molecular design of small molecule donors has led to a near doubling of the power conversion efficiency of solution-processed small molecule solar cells, with improvements from ~4 to ~8% (Figure 4A). Bazan and Chen’s work demonstrated that in addition to the initial design principles, small molecule donors should be constructed with greater than six π-conjugated units in the molecular backbone and decorated with sufficient numbers of alkyl side chains to ensure good solubility and uniform film formation from solution. In each case of high-performance donors, it should be noted not one molecule but rather a large series of compounds were synthesized and evaluated on their structure–property–function relationships; thus, the importance of a highly tunable synthesis cannot be overvalued. Since the report of PCEs in excess of 6% in 2012, the number of publications focused on the development of small molecule donors has increased exponentially (Figures 4B and 4C), with now numerous reports of devices giving PCEs in the range of 5–6%. As a final comment, while the spotlight has recently been directed on small molecule donors, an emerging area of interest is in the design and synthesis of linear donor–acceptor small molecule acceptors to replace fullerenes in BHJ solar cells.22 Such molecules can be pieced together from the current stock of building blocks used to construct high-performance donors and offer the potential of cost-efficient synthesis, photochemical stability, and increased photon harvesting (Figure 5). Efficiencies have reached 4% to date and are expected to continue to increase. It is fully expected that with the larger number of researchers in the field, and the wide range of building blocks available, a 10% efficient, solution-processed, all small molecule solar cell will be reported in the not-too-distant future.
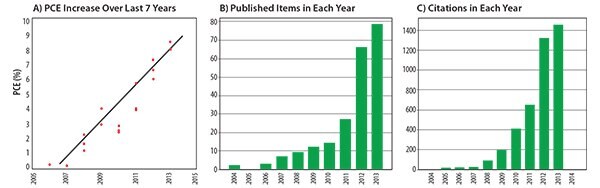
Figure 4.A) Plot of highest reported power conversion efficiencies for solution-processed small molecule solar cells from 2006 to 2013. Web of Knowledge generated reports showing B) the number of publications and C) the number of citations since 2003 for the search topic ‘solution processed small molecule solar cells’.
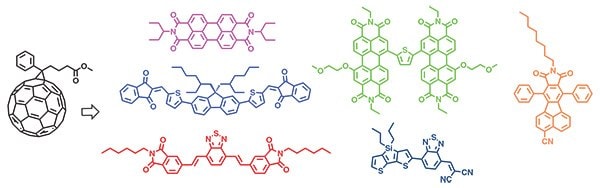
Figure 5.Example structures of linear donor-acceptor type small molecules utilized as acceptors replacing fullerene derivatives in solution-processed bulk-heterojunction solar cells.
Acknowledgments
Dalhouise University, NSERC, and the Canada Research Chairs program are thanked for financial support.
Related Products
References
To continue reading please sign in or create an account.
Don't Have An Account?