Segmented Copolymers of p-dioxanone, Glycolide, and Lactide: Advantages and Applications in Biomedical
Poly(p-dioxanone): Properties and Applications
Poly(p-dioxanone) (or PDS) is a polymer prepared via the ring-opening polymerization of p-dioxanone (Figure 1). PDS is used in a variety of applications including sutures, meshes, staples, clips, implantable orthopedic devices, and drug delivery applications.
/synthesis-of-poly(p-dioxanone).jpg)
Figure 1. General synthesis of poly(p-dioxanone) from p-dioxanone
Poly(p-dioxanone) has a low melting temperature (Tm=110 to 115oC), a low glass transition temperature (Tg=–10oC), and approximately 55% crystallinity. It also features in vivo absorption, low toxicity1,2, softness, and flexibility, in comparison to other biodegradable polymers. Due to its low Tg, ability to be crystallized, and biocompatibility, PDS is an ideal material for biomedical applications and medical devices, such as Ethicon’s PDSTM monofilament suture. Other examples of commercial devices composed of PDS homopolymer are shown below (Table 1):
When implanted or used in vivo, medical products or devices manufactured from PDS homopolymers are typically absorbed within six to nine months, making poly(p-dioxanone) a suitable material for slow-healing wounds.
Disadvantages of Poly(p-dioxanone) Homopolymers
Despite some physical advantages, poly(p-dioxanone) is not as thermally stable as other synthetic, absorbable polyesters as the monomer-polymer equilibrium favors the monomer. The shifted equilibrium requires modification of both synthetic and processing procedures compared to other resorbable materials. To prevent depolymerization, poly(p-dioxanone) must be processed at the lowest possible temperature. Additionally, during synthesis by solid–state polymerization, the reaction temperature must be kept below the melting point of the forming polymer and unreacted residual monomer is removed by volatilization under vacuum at temperatures below the melting point of the polymer. Additionally, the use of PDS homopolymers in absorbable medical devices is fairly limited due to relatively long absorption time and limited photo and thermal stability. To expand the utility of p-dioxanone as a monomer, segmented copolymers of p-dioxanone with glycolide or L-lactide were developed.
Segmented p-Dioxanone: Glycolide Copolymers
Segmented copolymers of p-dioxanone and glycolide (Figure 2) combine the fast absorbing characteristics of polyglycolide with the flexibility of poly(p-dioxanone), yielding a product with more ideal properties for certain biomedical applications4.
/structure-poly(p-dioxanone-co-glycolide).jpg)
Figure 2.Structure of poly(p-dioxanone-co-glycolide)
Sutures are typically characterized by composition, suture structure, absorption time, breaking strength retention (BSR). When measured in vivo, breaking strength retention is a measure of time in which the material does not undergo critical failure under tension in vivo3. While this measurement is specific to suture materials, the results can be extrapolated to the general longevity and stability of the material in vivo, regardless of the application. PDS has typical absorption time of six to nine months and BSR profile of approximately six to eight weeks. The addition of glycolide as a comonomer (up to 15 mol), resulted in a reduced absorption time of three to four months and a reduced BSR profile of three to four weeks (Figures 3 & 4).
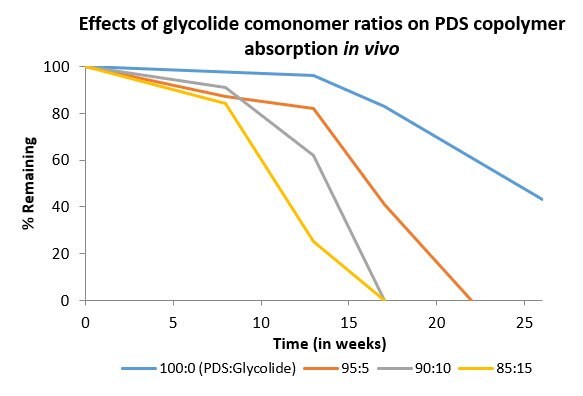
Figure 3. Effects of monomer composition on in vivo absorption of p-dioxanone:glycolide copolymers
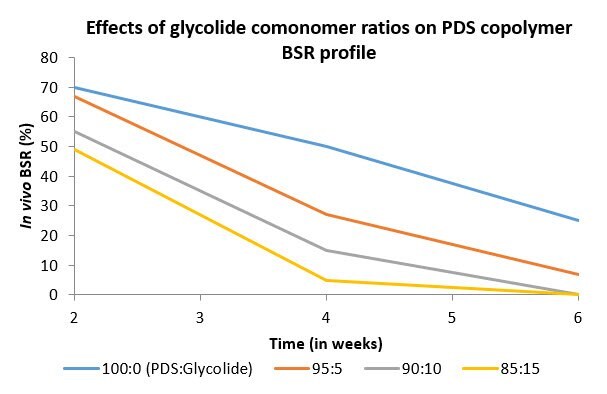
Figure 4. Effects of monomer composition on in vivo breaking strength retention of p-dioxanone:glycolide copolymers
Additionally, minimal effects were seen on tensile properties, crystallinity, and thermal properties, in comparison to PDS homopolymers (Table 2).
These copolymers feature an ideal combination of mechanical properties, fast absorption, and excellent pliability, making them useful in a variety of biomedical applications.
Segmented p-Dioxanone: L-lactide Copolymers
Segmented copolymers of p-dioxanone and L-lactide (Figure 5) feature improved pliability in contrast to PDS homopolymer and have similar handling properties to ε-caprolactone:glycolide copolymers5. With the increased hydrophobicity of L-lactide, due to the addition of the methyl group on each repeat unit, the rate of absorption in vivo is similar to that of PDS homopolymers. Moreover, the addition of up to 15 mol% L-lactide had a minimal effect on tensile properties, crystallinity, and thermal properties.
/structure-poly(p-dioxanone-co-lactide).jpg)
Figure 5. Structure of poly(p-dioxanone-co-lactide)
Conclusion
With the addition of glycolide or lactide as comonomers, polymers with a range of observed in vivo absorption and pliability can be developed. By controlling the ratio and identity of monomer, copolymers can be synthesized to match the properties needed for a specific application. Breaking strength retention can be controlled by altering the ratio of p-dioxanone to glycolide, where handling properties can be improved by controlling the ratio of p-dioxanone to L-lactide. Proper selection of p-dioxanone comonomers and their ratios will result in a copolymer featuring the desired in vivo absorption and necessary flexibility.
Materials
References
To continue reading please sign in or create an account.
Don't Have An Account?