Effectiveness of Higher Volumes and Sequential Sampling in Active Air Monitoring
The new 2020 draft of EU GMP Annex 1 regulates the manufacture, control and release of sterile pharmaceutical products in the EU and gives more importance to quality risk management (QRM) than its current version. Hence there is a need to know more about the manufacturing processes used and the microbial contamination risks of the manufactured product. This study investigates the feasibility of modifying the most frequently used active air sampling method in two different ways to enhance the knowledge about these risks by
- Increasing the volume of sampled air
- Sampling onto the same plate at regular intervals
An environmental monitoring plan must include the determination of sampling locations, monitoring frequencies, and monitoring methods. Incubation conditions should be based on a documented risk assessment and knowledge of the process and operations to be performed in the area. The specific limits or frequencies specified in the draft should be considered as the minimum requirement.
- The Objective of Active Air Sampling Study
- Materials and Methods: Microbial Air Sampler and ICR Settle Plates
- Results of High Volume and Sequential Sampling
- Conclusion of the Active Air Sampling Study
- Materials
Modified methods in tests with the MAS-100 NT® air sampler and ICR settle plates

The well-known environmental monitoring methods based on volumetric air samplers, settle plates, contact plates, and swabs are recommended to be applied frequently. In general, there is no need to change the well-established procedures, which have been proven to be efficient. To achieve continuous viable air monitoring, it is recommended to use settle plates in combination with active air samplers.
Newly included rapid or automated methods can fulfill the requirement to perform continuous airborne viable monitoring, but the 2020 EU GMP Annex 1 draft states the limits in colony forming units (CFUs). When different or new technologies generate results in units other than CFUs, the manufacturer should scientifically justify the limits set in another unit and, wherever possible, correlate them with colony counts. However, it is mandatory to validate and demonstrate the equivalence or superiority of these methods over the established methodology. Therefore, it is worthwhile to look at alternatives to such real-time methods.

One such method is sequential sampling, which uses air samplers in combination with culture media, like the conventional method but covers a longer period of the manufacturing process. The MAS-100 NT® air sampler offers the built-in option to perform sequential sampling, meaning a determined air sample volume of up to 2000 liters is taken in up to 50 intervals for up to 24 hours, during which the same settle plate is used every time. There’s no need to change the plate thereby avoiding secondary contamination and thus reducing the risk of false-positive results.
The Objective of Active Air Sampling Study
- To determine if dehydration of the culture medium impacts the accuracy of results when larger volumes of up to 4000 liters of ambient air are sampled (Part 1).
- To compare the effectiveness of conventional and sequential (interval) sampling by counting the CFUs after the incubation period (Part 2).
Active air sampling was performed in a non-controlled environment with intense human activity to detect a broad range of airborne microorganisms and, furthermore, to achieve higher microbial counts than in a controlled environment.
Materials and Methods: Microbial Air Sampler and Ice Settle Plates
Newly calibrated MAS-100 NT®microbial air samplers were used in combination with TSA with LTHThio sedi. -ICR settle plates (same batch of all trials) for all sampling.
Settle plates were dried by a MAS-100 NT® air sampler prior to sampling to simulate the influence of a larger air volume on the water loss of these media. This was done under a laminar flow hood to avoid contamination and enable only the colony counts of the air drawn during the subsequent sampling step. Half of the plates were incubated immediately after the initial drying step to serve as negative controls to establish the cleanness of the air in the laminar flow hood. All air sampling was performed in a non-controlled area and started with a 1-minute delay to avoid contamination caused by the presence of the lab technician.
Relative humidity and temperature were recorded with a calibrated instrument during sampling to evaluate the effects of these factors on water loss. The colonies of microorganisms on the agar media were counted after 1 or 3 days of incubation and expressed as colony forming units (CFUs).
Part 1: Increasing air sample volumes up to 4000 liters
The aim of this part of the study was to measure drying of the agar media as a function of the sampled air volume.1
Four MAS-100 NT® microbial air samplers were used at sampling locations positioned about three meters apart. The reference and test plates alternated, and the order was changed after every run. This ensured that every air sampler with different type of plate would sample air at every location.
The test procedure is shown in Figure 1. The first step was to dry plates, with half of the plates used as negative controls and the other half as test plates. The second step was to perform side-by-side tests with pre-dried test plates and reference plates without prior drying. The reference plates (fresh plates, not pre-dried) served to determine if water loss of the pre-dried plates had led to lower recovery rates.
The water loss of each plate was determined by weighing before and after the initial drying step, as well as at the end of the subsequent sampling procedure. For initial drying, two different air sample volumes of 2000 or 3000 liters were applied per plate. As the air sampling volume for the test added 1000 liters per plate, the total air volume per test plate was 3000 or 4000 liters (Table 2). For each selected sample volume 10 runs were performed in parallel in a side-by-side test. After sampling, the plates were incubated at 30 to 35 °C for 1 to 3 days. The colonies on the settle plates were counted and the values corrected according to Feller statistics.
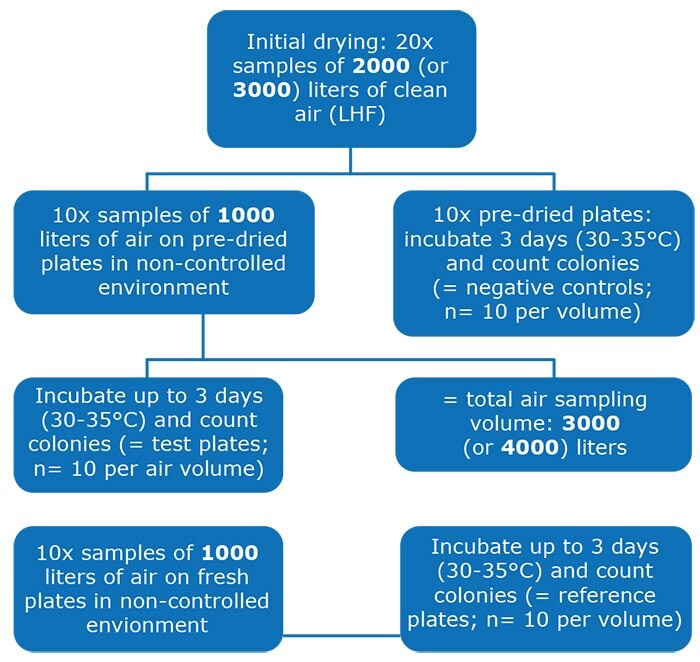
Figure 1.(Test procedure for Part 1: For each sampling volume 20 plates were pre-dried (e.g. for the total air volume of 3000 liters, 20 plates were pre-dried with 2000 liters)
Part 2: Conventional and sequential (interval) sampling method
In conventional air sampling a specific air volume is sampled at a certain time onto a different agar plate for each measurement. However, in sequential air sampling, several samples are taken at regular intervals over a certain period using the same plate1. The interval was 30 minutes, and the total measurement period was 2 hours.

Figure 2.(Photo of the three air sampling locations for Part 2. The distance between the sampling locations was approx. three meters)
Inactivity and active air sampling alternated for sequential sampling. Over 2 hours, 1000 liters of air were sampled, 200 liters at the start and subsequently 200 liters every 30 minutes. Conventional sampling was performed at the same intervals, but the plates were exchanged for new ones every time, and the sampling volume was at 1000 liters each (Table 1).
All agar plates were pre-dried under the laminar flow hood with an air volume of 1000 liters. At the end of the experiment, the total sampling volume was 2000 liters/plate. The relative humidity was 44% on the day of the experiment. The CFUs were counted after a 3-day incubation at 32.5 °C.
According to the product specifications, each settle plate is filled with 30 mL of agar medium weighing about 30 grams. A weight loss of, for example, 6 grams was a 20% weight loss, and thus a 20% loss of water. Plates were weighed with the Petri dish lid. The water loss per agar plate is shown in Figure 3.
As expected, the plates at the higher humidity of 52 to 54% during drying and sampling (series 3 and 4) lost less weight and thus less water than those at the lower humidity of 37 to 38% (series 1 and 2), see Figure 3. At both humidity values, the water loss was almost linearly proportional to the total air volume drawn, with the rate of water loss slowing only very slightly as the air volumes increased.
Figure 3.Average water loss of the settle plates' agar media in grams at different total air volumes for series 1/2 (left) and 3/4 (right)
The CFU counts of the reference plates and the pre-dried plates of the same series were very similar (Figure 4). This indicates that colony formation was not impaired by the water loss. Had water loss impaired colony formation, the fresh reference plates would have yielded significantly higher counts than the corresponding pre-dried plates.

Figure 4.CFUs per fresh reference plate and 2000 or 3000 liter pre-dried plate for series 1/2 (left) and 3/4 (right) after a one-day incubation at 32.5 °C.
The different absolute levels of total CFUs between series 1/2 and series 3/4 are due to differences in the number of microbes in the air on the days the sampling took place. This is evidenced by the similar count levels of the corresponding reference plates.
Figure 5 illustrates the number of colonies counted in the pre-dried plates as a percentage of those on the reference plates, as well as the amount of water lost after initial drying and after air sampling. Following the drying step, water loss is calculated as the weight lost after drying/sampling. It shows that the numbers of recovered colonies on the pre-dried plates did not differ significantly from those of the reference plates without pre-drying, despite a significant loss of water (e.g., 2000 L series 1: 98% recovery rate and 25% total water loss; 2000 L series 3: 93% recovery rate and 20% total water loss).


Figure 6.Settle plate with shrinkage at the edges of the agar
In five out of the ten plates in series 2 (3000 liters pre-drying volume plus 1000-liter air sampling at 37 to 38% relative humidity), the agar had shrunk after a two-day incubation at 32.5 °C (Figure 6). No agar shrinkage was visible after 24 hours of incubation at 32.5 °C or after two days where the relative humidity had been a higher 52 to 54% (series 4, Figure 3). This underlines the relevance of the environmental conditions (temperature and relative humidity) during sampling (see series 4).
Part 2: Conventional and sequential (interval) sampling
Sequential and conventional sampling were compared with regards to the suitability of these methods to recover colonies on settle plates, each after a 1000-liter initial drying step in a laminar flow hood and a subsequent 1000-liter sampling step in the non-controlled environment. The average numbers of colonies recovered per cubic meter of sampled air are shown in a boy-blot illustration (Figure 7). This indicates that sequential and conventional sampling yielded similar colony counts.
All plates were weighed before and after the pre-drying step, and again after the side-by-side test procedure. The water loss during pre-drying and sampling had amounted to between 4.74 and 5.39 grams per plate, which is equivalent to 15.8% and 17.9% of the agar media's weight.
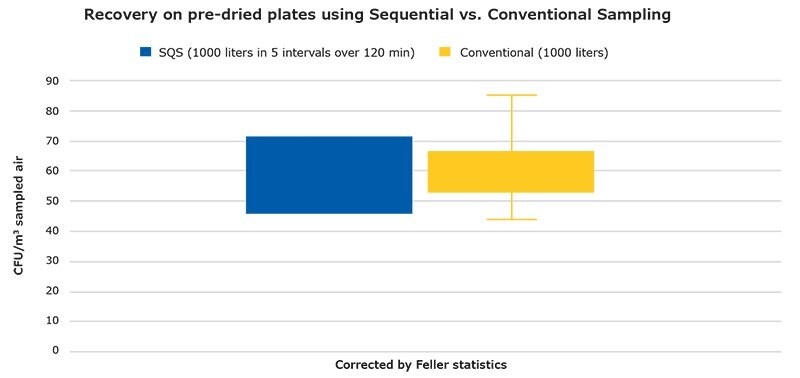
Figure 7.CFUs per m3 of sampled air for conventional and sequential air sampling (SQS) at the 3 collection points (see figure 2 and table 2 for the test set-up). The SQS value is the average count of the two SQS locations.
Conclusion Of The Active Sampling Study
By adding a long drying step before sampling with the air volume of 1000 liters, it was possible to simulate the influence of sampling of up to 4000 liters on the water loss of TSA settle media plates used in combination with the MAS-100 NT® microbial air sampler, as well as the effects it has on microbiological detection. The test results reveal an almost linear water loss over sampling time (and over air volume). However, in the air volume range tested, the significant water loss caused by evaporation was shown not to impair the ICR settle plates' suitability for microbiological detection. Hence it is possible to sample up to 4000 liters without impacting the quality of results, allowing longer and/or more comprehensive active air sampling in manufacturing facilities. The simultaneously processed undried reference plates suggested that the determined higher or lower absolute colony counts were due to different contamination levels on the days of the experiment.
The objective of the second set of experiments was to determine whether sequential air sampling would lead to different colony counts than conventional sampling. In sequential air sampling, the air sampler is programmed to sample air on a single agar plate at certain intervals over a pre-defined period. This period can cover a whole working process during which, unlike for conventional air sampling, it would not be necessary to replace the agar plate several times, avoiding the risk of contamination on the plate (and thus false-positive results) caused by the manual act of plate replacement. The comparison revealed that the result quality of sequential air sampling is comparable to that of the conventional sampling method, suggesting that this alternative method may be preferable for manufacturing activities over a longer period during the course of a day.
References
To continue reading please sign in or create an account.
Don't Have An Account?