Fluorescent Retinoid Probes

Fluorescent retinoic acid analogues for use in cellular imaging, protein-ligand binding assays, and flow cytometry
Introduction to Fluorescent Retinoid Probes
LightOx fluorescent retinoid probes (Figure 1) are a family of synthetic analogues of all-trans-retinoic acid (ATRA) that exhibit strong solvatochromatic fluorescence when excited with UV/violet light.1 In a range of biological assays, LightOx fluorescent retinoid probes display comparable and, in some cases, more potent biological activity than ATRA and can therefore be used as effective replacements for ATRA in a broad range of assays, especially those involving retinoic acid receptors (RARs).2–5 LightOx17 (Figure 2) is longer than the other analogues and does not exhibit retinoid activity and is supplied as non-bioactive fluorescent probe with similar characteristics to the other retinoid derivatives.
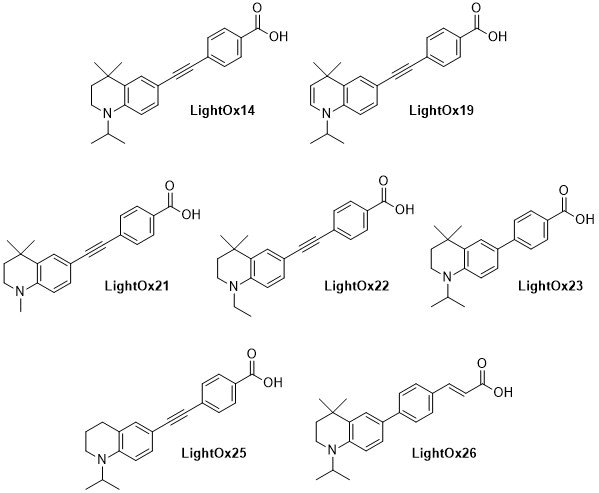
Figure 1.Chemical structures of LightOx fluorescent retinoid probes.
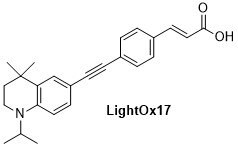
Figure 2.Chemical structure of non-bioactive LightOx fluorescent retinoid probe.
Photophysical properties
LightOx fluorescent retinoid probes can be excited by typical UV-A (e.g. DAPI filter set) and violet light sources (405 nm). The fluorescence emission is readily detected in a cellular environment using a fluorescence microscope, in cuvette-based experiments using a spectrophotometer, and in a well plate format using typical plate readers.
The absorption and emission characteristics are highly solvatochromatic (Figures 3-4). In particular the emission wavelength and intensity is highly dependent on the local environment: intense and blue-shifted in non-polar environments and weaker and red-shifted in polar environments. In microscopy experiments, for example, this enables the acquisition of environmental information in addition to localization behavior.1
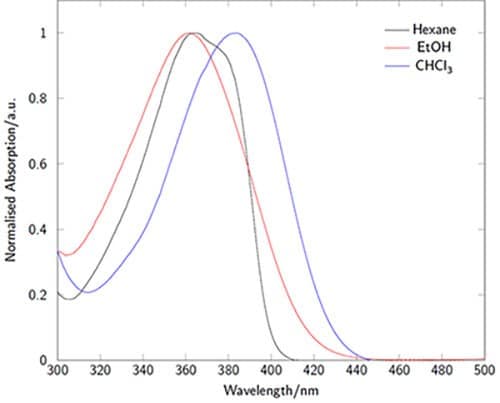
Figure 3.Absorption spectra of LightOx14 in a variety of solvents.
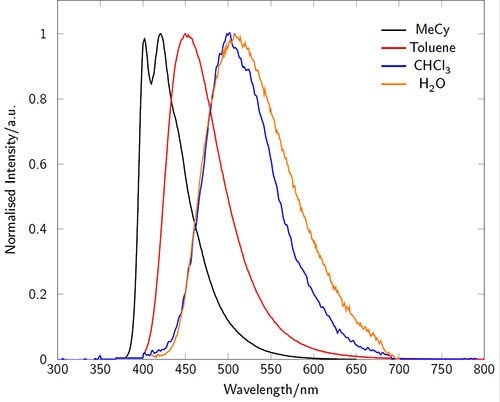
Figure 4.Fluorescence emission spectra of LightOx14 in a variety of solvents.
Retinoid Protein Binding Properties
The majority of the LightOx fluorescent retinoid probes exhibit high affinity binding for retinoid-binding proteins, including the retinoic acid receptors (RARs) and cellular retinoic acid binding protein II (CRABPII) (Table 1) with the exception of LightOx17, which is a non-RAR binding control. This strong binding can be utilized for the development of competitive fluorometric binding assays, wherein the displacement of the fluorescent retinoid from a protein target can be monitored via fluorescence emission and used to determine the relative binding affinity of a competing ligand.
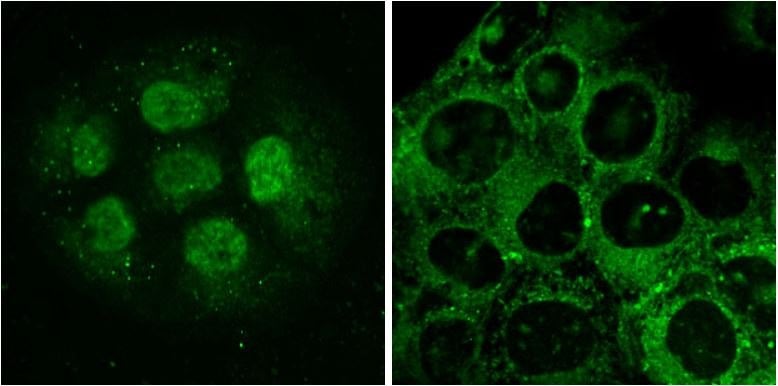
Figure 5.LightOx14 live-cell labeling of HaCaT epidermal keratinocytes. Cells in left and right panels were labelled for 60 minutes prior to imaging. Left: Nuclear labelling with LightOx14 detected in small clumps of proliferative cells. Right: LightOx14 detected in cytoplasmic and nuclear compartments in partially differentiated cells.
Preparation Instructions
LightOx fluorescent retinoid probes are highly soluble in DMSO and can be prepared as a stock solution up to a concentration of 10 mM.
Storage and Stability
LightOx fluorescent retinoid probes are light-stable and require no special precautions when used. Solutions in DMSO should be stored between 4 to -20 oC in the dark and have a shelf-life of around 6 to 12 months.
Safety and Disposal
Samples should be treated with bleach prior to disposal.
Materials
References
Contact Information
For the most recent information please visit https://lightox.co.uk/imaging-probes/.
To continue reading please sign in or create an account.
Don't Have An Account?