Improving API Solubility with Mesoporous Silica
The poor solubility of active pharmaceutical ingredients (APIs) can limit the bioavailability of otherwise promising drugs, necessitating the application of tailored strategies to overcome this challenge. These strategies include optimizing API characteristics early in development through API processing and employing advanced formulation techniques, such as spray drying, hot-melt extrusion, and inorganic drug carriers, to improve dissolution performance and API solubility.
What is Mesoporous Silica?
Mesoporous silica, a highly porous form of silicon dioxide, which can be used as an inorganic drug carrier to stabilize the more soluble, amorphous form of APIs within nanosized pores. When a concentrated API solution is added to mesoporous silica, the API is adsorbed onto the surface of the silica and confined within the pores, effectively preventing re-crystallization and enhancing API dissolution. This process of pore adsorption and nanoconfinement enables formulation with mesoporous silica.
Figure 1 outlines this process using Parteck® SLC mesoporous silica as the solubility-enhancing excipient. Specially designed with a very large surface area of 500 m2/g and small 6 nm pores, Parteck® SLC excipient enables optimal stabilization of APIs even at high drug loads. The use of this mesoporous silica also facilitates the formulation of challenging APIs, such as poor glass formers that are unstable in their amorphous form and where other solubility-enhancing strategies prove ineffective.
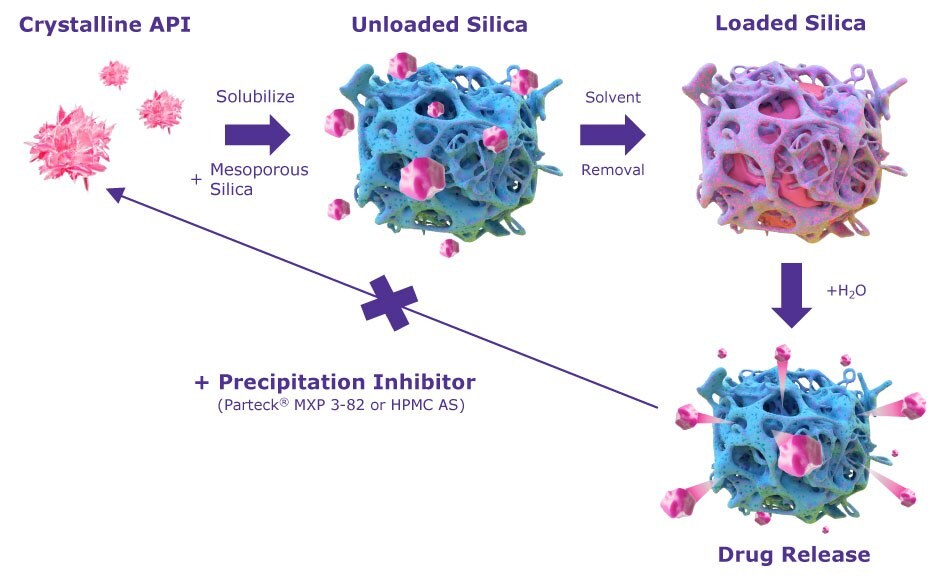
Figure 1.Mode of action for Parteck® SLC inorganic drug carrier. The more soluble, amorphous form of an API is first adsorbed and stabilized in the nanosized pores of mesoporous silica, followed by release of the API.
Advantages of Mesoporous Silica
Parteck® SLC mesoporous silica offers a robust solution for addressing solubility challenges in drug development, particularly for APIs with poor glass-forming ability or unstable polymorphism. By loading the API onto the surface of the mesoporous silica material, a consistent and stable solid-state profile can be achieved, ensuring reliable formulation and optimized pharmaceutical performance. It's GRAS (Generally Recognized as Safe) and multi-compendial status provide assurance of safety and compliance.
Key benefits of Parteck® SLC mesoporous silica include:
- Enhanced solubility: Improves API solubility by adsorbing and releasing APIs in a reproducible manner through its unique mesoporous structure
- Unmatched stabilization: Maintains the amorphous solid state, reducing the risk of re-crystallization up to a drug load of 50%
- Platform versatility: Effectively manages a variety of APIs and lowers the risk of polymorphism and morphological changes during drug development
- Advanced material properties: Features a unique large surface area and a small pore diameter, resulting in a high drug loading capacity and user-friendly particle characteristics
- Improved processability: Loading the API onto the silica excipient provides a consistent and reliable solid-state profile, reduces the risk of unstable polymorphism, and enhances API flowability, compressibility, and particle homogeneity
Find a comparison of inorganic drug carriers such as mesoporous silica and other solubility-enhancing formulation strategies in our technical article “API Solubility and Dissolution Enhancement Via Formulation".
Loading Mesoporous Silica
The wet impregnation process for loading APIs into mesoporous silica is a precise and effective method for addressing solubility and stability challenges in drug development. The key steps include dissolving the API in an organic solvent, loading the API onto the mesoporous silica, and then removing the solvent. An overview of important considerations for the loading process is shown in Table 1.
Preparing the API Solution
Preparation of the API solution requires optimization of several parameters. Suitable organic solvents must be selected to ensure chemical compatibility and adequate API solubility. The solution stability should be assessed, particularly when elevated temperatures are needed for dissolution, to prevent degradation or chemical changes. Additionally, achieving a balance between solvent volume and API concentration is essential for effective loading and manageable process conditions that involve solvent removal.
Loading the API Solution
During the loading step, the prepared API solution is evenly distributed onto the mesoporous silica bed. Key parameters include controlling the feed flow rate and droplet size for uniform coverage without over-wetting, maintaining optimal temperature and agitation to support solvent evaporation and even distribution, and balancing solvent evaporation rates to ensure API penetration into the silica pores while preventing recrystallization.
Removing Residual Solvents
The drying phase is crucial for removing residual solvents and finalizing the API’s solid-state profile. Key parameters include optimizing temperature, pressure, and agitation to ensure efficient solvent removal while preserving the amorphous state of the API and minimizing drying time. A nitrogen sweep aids in maintaining a controlled environment, and while continuous drying is preferred, alternating loading and drying cycles may be used to prevent over-wetting and improve process yield.
Loading Mesoporous Silica
As illustrated in Figure 2, various loading methods exist, offering a high flexibility for both lab-scale and production-scale applications, whether solvent-based or solvent-free, batch or continuous manufacturing processes are preferred.
Wet impregnation is the most commonly used method, as spray dryers and high-shear extruders are typically available in both small-scale lab and large-scale manufacturing settings. For initial trials using solvent-based methods, no additional capital investment and or specialized equipment is required, as lab-scale loading can be performed using common lab equipment through a suspension or impregnation method.
Furthermore, employing Parteck® SLC excipient in a hot-melt extrusion process addresses the limitations associated with organic solvents in solvent-based loading, while still harnessing the benefits provided by Parteck® SLC silica. If avoiding organic solvents is essential, initial trials to assess suitability can be conducted using small-scale extruders or a ball mill.
Explore our experimental loading guide.
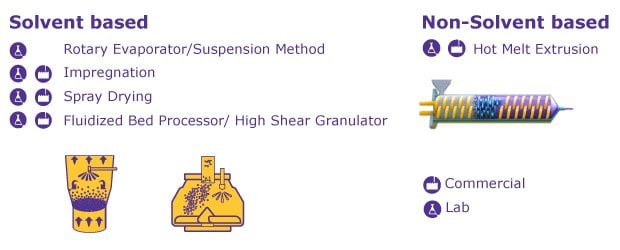
Figure 2.Solvent and non-solvent based methods for loading mesoporous silica.
Evaluating the Final Product
After completing the wet impregnation process, the bulk material undergoes rigorous characterization to ensure it meets pharmaceutical quality standards and provides desired, stable characteristics:
- Solid-state profile: Absence of crystalline material and stable amorphous state
- Chemical analysis: Conforms to expected assay and chemical profiles
- Homogeneity: High uniformity of the loaded API
- Residual solvent content: Ensure levels are below International Council for Harmonization (ICH) limits1
- Stability: Confirmed using XPRD and DSC
- Performance metrics: Improved dissolution and reliable solid-state properties
Scalability
When optimized, the wet impregnation process provides a scalable platform for consistent API delivery. For small-scale loadings of 1–200 g, simple lab equipment can be used, while suitable equipment for loading and drying can be used for development-scale loadings in the range of 1–13 kg. Ultimately, the process can be scaled up to at least 500 kg loadings in a production setting without requiring further process development.
Wet Impregnation Process
The wet impregnation process is scalable from small to production scale.
Small Scale

Development Scale
Production Scale
Studies described in the webinar “Solubility Enhancement, Stability, and Scalability of Mesoporous Silica Formulations: From Lab to Production Scale” confirm consistent stability of the amorphous form and dissolution performance in the final dosage form of an API at lab and production scales.
Correlation of In Vitro and In Vivo Dissolution Profiles
Mesoporous silica-based dosage forms that improve in vitro dissolution can improve the bioavailability of poorly soluble drugs in vivo by facilitating rapid dissolution. A peer-reviewed study compared the in vitro and in vivo dissolution of mesoporous-based fenofibrate formulation.2 The API was loaded onto Parteck® SLC mesoporous silica using the wet impregnation method, achieving a target drug load of 30% amorphous stability throughout the time of investigation.
The in vitro dissolution tests showed significant solubility enhancement and supersaturation for both the suspension and capsule samples. Although the capsule samples exhibited a noticeable lag time in dissolution, the area under the curve remained comparable. The in vivo studies in fasted pigs confirmed the enhanced solubility and bioavailability of the fenofibrate-Parteck® SLC formulation, with both capsules and suspension significantly surpassing the crystalline compound in area under the curve (Figure 3).
The stability of the amorphous form was still present after 12 months of storage in three different conditions (cold, ambient, and accelerated). The dissolution profile showed the same performance regardless of the storage condition or immediately after loading (data not shown).
The significant enhancement in fenofibrate dissolution achieved with the mesoporous silica-based formulations and the correlation of in vitro and in vivo results demonstrate the effectiveness of this strategy in addressing solubility challenges.
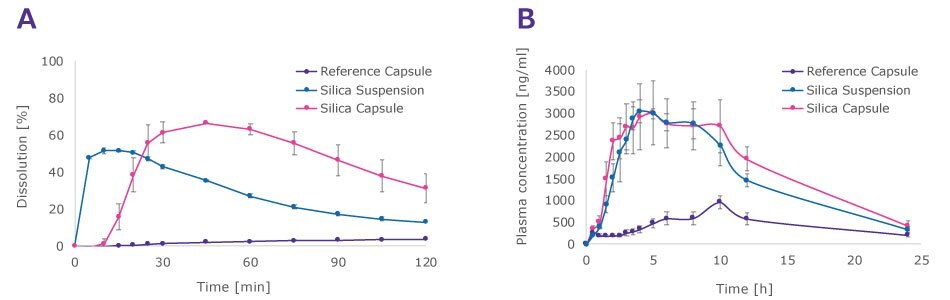
Figure 3.A) In vitro and B) in vivo dissolution profiles of fenofibrate, comparing API-loaded Parteck® SLC material filled in a capsule (reference capsule), API-loaded Parteck® SLC material suspended in water (reference suspension), and crystalline API (= reference), each blended with 12.5% precipitation inhibitor.
In vitro dissolution method: tests (n=3) were carried out in a USP Type II apparatus, 500 mL FaSSIF, 37 ± 0.5 °C, 75 rpm, 290 nm, n=3; in vivo bioavailability studies in fasted, male Landrace pigs, mean weight 14.5 kg, n=6.
The Advantage for Poor Glass Former APIs
Amorphous stability is a key challenge during the development of poorly soluble drug formulations. Glass-forming ability is a key physicochemical parameter that can predict long-term amorphous stability. Amorphous solid dispersions (ASDs) in polymeric matrices such as spray-dried dispersions or hot-melt extrusions aim to prevent recrystallization by suspending drugs in a polymer mesh. For good glass formers (GFA Class 3), this approach effectively stabilizes the amorphous state.
In contrast, a compound with a poor glass-forming ability (GFA Class 1) is fragile in the amorphous form and tends to crystallize. This characteristic puts poor glass former APIs at a relatively higher risk of failure in commercial development as they are not readily stabilized by polymer-based technologies due to their molecular mobility in polymers. In addition, these molecules are often excluded from an API screening approach, preventing a full exploration of their potential efficacy.
With its high surface area of 500 m2/g and 6 nm pores, Parteck® SLC silica is an ideal option for improving the solubility of APIs with poor glass-forming ability. The more soluble, amorphous form of poor glass formers is stabilized due to their steric confinement in the nanosized pores of the silica which reduces molecular mobility, even at high drug loads.
This formulation strategy for stabilizing poor glass former molecules outperforms formulations of amorphous solid dispersions and is an effective and easy way to enhance and stabilize this class of molecules.3
For additional insights into how Parteck® SLC serves as a versatile platform technology, watch our on-demand webinar “The Importance of Amorphous Stability: Mesoporous Silica for Poor Glass Formers”, where we explore crystal structure, polymorphs, and how our mesoporous silica solution effectively mitigates polymorph variability in drug development.
Additionally, our brochure "Adsorb, Stabilize and Enhance" details how Parteck® SLC mesoporous silica enhances solubility for challenging compounds, such as poor glass formers.
Parteck® SLC Mesoporous Silica as a Platform Approach
Unexpected polymorphic changes of an API can significantly extend development timelines and require investigations to determine the source of variation. Such polymorphic variation can significantly extend drug development timelines, as a thorough understanding is required to design a manufacturing process that mitigates its impact. Failure to do so may result in unexpected polymorphic transformations at production scale or during storage, potentially affecting the drug’s quality, performance, and manufacturability due to variations in physicochemical and galenical properties. Regulatory authorities such as the ICH and FDA mandate polymorph screening as a critical component of drug development. If multiple polymorphs are identified, the most favorable form must be stabilized, as amorphous polymorphs, while typically more soluble, are not thermodynamically stable.
Using Parteck® SLC mesoporous silica as drug carrier effectively addresses this challenge. Loading the inherently unstable amorphous API onto the drug carrier stabilizes the API by sterically hindering the drug molecules, significantly limiting their molecular mobility. This stabilization prevents the amorphous API from converting into a more thermodynamically stable, yet less soluble crystalline form, during storage, a common issue with solid dispersion formulations. This strategy is applicable to a broad range of APIs, serving as a platform technology that enhances formulation development efficiency.
Read our white paper “Use of a Platform Formulation Technology to De-Risk Solid-State Variation in Drug Development” or our brochure “Drug Substance, Meet Drug Product” for additional insights into how Parteck® SLC excipient stabilizes diverse APIs, ensuring consistent formulation development and efficient manufacturing processes by effectively managing particle and polymorph variation.
Conclusion
Addressing the persistent challenge of poorly soluble APIs is essential for unlocking the therapeutic potential of innovative drugs. Parteck® SLC mesoporous silica stands out as a transformative platform technology that not only enhances solubility but also stabilizes the amorphous form of APIs, effectively mitigating the risks associated with unstable polymorphism. With its advanced material properties – such as a high surface area and nanosized pores – Parteck® SLC mesoporous silica delivers unmatched stability and processability, even for APIs with poor glass-forming ability.
By integrating mesoporous silica into their formulations, pharmaceutical developers can achieve consistent, scalable results, that expedite identification of more reliable formulation development. Whether it’s improving bioavailability, ensuring stability, or accommodating diverse API profiles, Parteck® SLC mesoporous silica offers a versatile and effective solution for overcoming solubility and stability challenges in drug development.
Looking to the future, this platform technology holds great promise to expand the formulators’ toolbox, empowering them to overcome undesirable API characteristics such as poor flowability, low aqueous solubility, and unstable polymorphs.
We invite you to explore the potential of Parteck® SLC mesoporous silica in your formulations and benefit from the difference it can make in advancing your drug development projects.
Need expert guidance? Reach out to our specialists to explore formulation solutions.
Related Products
References
To continue reading please sign in or create an account.
Don't Have An Account?