Scalability Challenges of Viral Vector Manufacturing
As the cell and gene therapy market continues to grow at a rapid pace, viral vectors have become the most reliable and sought-after method for gene delivery. This is due to the flexibility they offer in delivering new genetic material into target cells, as well as other advantages, such as low immunogenicity and high transduction efficiency of target cells, which can lead to high in vivo expression levels of the introduced gene of interest.
The most used viral vectors, adeno-associated virus (AAV) and lentivirus, have been produced historically in adherent cell culture using generic, unoptimized, and undefined media formulations, which are typically supplemented with fetal bovine serum. However, challenges with this process can lead to limited cell growth and lower overall viral vector productivity, and to the introduction of adventitious agents from animal-derived media.
To meet the ever-increasing demand for cell and gene therapies (CGTs), the industry must shift away from this inefficient and risky approach and adopt fit-for-purpose tools that enhance upstream operations.
Challenges Of Traditional Cell Culture Methods
Many of the legacy processes and technologies can efficiently produce enough viral vectors for the smaller batch sizes needed in the early days of gene therapy. However, these methods fall short in meeting the process economics and scalability demands of viral vectors when expanding to larger patient populations. Just as the industry rallied to improve productivity for monoclonal antibodies (mAbs) through new, innovative tools and techniques, the same needs to be done for CGTs and their key manufacturing component, viral vectors.
The challenges of traditional adherent systems include:
- Adherent growth-dependent cell lines
- Use of serum
- Scaling out (increase in identical units)
- Labor intensive
- Open processing
- Higher cost due to labor, overhead, and raw materials
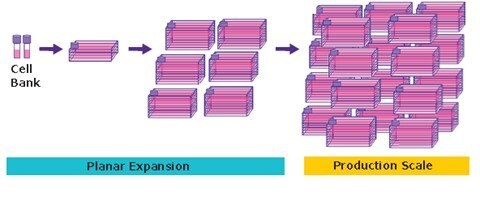
Figure 1.Adherent cell culture begins with a cell bank that undergoes planar expansion before production scaling in a large number of flasks.
Adherent Cell Culture Limit Scale and Increase Contamination Risk
Adherent cell culture production processes can pose challenges to the safety and quality of the vector. One challenge is that it utilizes tissue culture flasks, CellStack® devices, or roller bottles, which can limit scale due to a small amount of surface area or facility area. Due to the sheer number of vessels that need to be processed for even smaller clinical batches, processing becomes laborious and limits the maximum practical batch size (Figure 1). The process usually requires multiple rounds of manual handling and manipulation of cell cultures within a biosafety cabinet, increasing the risk of contaminants due to the open processing.
Animal Origin Components
Traditional methods also rely on undefined media, including components such as hydrolysates and/or sera, that contain growth factors and other nutrients to stimulate cell growth. The most frequently used serum, fetal bovine serum, provides a robust culture system but, due to its animal origin, introduces adventitious agent risk into the process and the final product. Additionally, the lot-to-lot variability inherent to sera contributes to inconsistency in cell culture performance and viral vector productivity.
As the CGT market evolves, regulatory agencies are focused on adapting their requirements in tandem with scientific and clinical breakthroughs, potentially resulting in increased stringency in expectations and processes. For example, the biopharmaceutical industry continues to move away from riskier components, such as animal-derived media, as new technologies are developed. This is reminiscent of the mAb evolution when CHO cells moved to the forefront of recombinant protein production only after being grown in suspension using animal origin-free media.
Serotype Challenges for AAV Vectors
Another challenge when producing AAV is there are many serotypes with unique tropisms from which to choose. Each serotype has its own biology; some serotypes require high production volumes and produce low vector yields.
Empty Capsids Reduce Efficacy of Final Drug Product
Finally, viral particles that do not contain packaged genetic material (i.e., empty capsids) are also produced during this process. This is a challenge as the empty capsids can reduce the efficacy of the final drug product. Therefore, alternative methods for cell culturing are necessary to meet today’s viral vector demand.
Suspension Cell Culture System for Large Batch Processing
Adherent production processes are amenable to scale-out (increase in number of identical units) for large scale production but not for scale-up (increase in vessel size). A suspension culture system, where cells grow suspended in a liquid medium, offers a viable alternative to adherent culture systems.
The advantages of a suspension culture system include:
- Suspension-adapted cell lines
- Chemically defined medium/feeds and virus production boosting agents
- Scale-up in bioreactors (increase in vessel size)
- Less labor intensive
- Shift towards closed processing
- Lower costs
As suspension culture systems can be scaled to larger batches while also yielding significantly higher doses per single batch. A major benefit of suspension culture is the ability to grow the cells in a chemically defined, animal component-free medium, thereby removing animal containing components, which reduces safety risks and overall process variability.
Adaptation of cells to non-adherent growth is a prerequisite for a switch from adherent to suspension-based cell culture and virus production. HEK293T cells can be adapted to grow in single cell suspension in an animal component-free, chemically defined medium (CD Medium) (ex: Cellvento® 4HEK Liquid and EX-CELL® CD HEK293 Viral Vector Medium) where all components are known. Since CD Medium does not contain hydrolysates or sera, this increases consistency in product performance with respect to cell culture growth and production of lentivirus. The use of CD Medium also reduces regulatory burden due to a lack of animal origin materials.
Once the cells are adapted to growth in suspension mode in CD Medium, process development in bioreactors can proceed. Growth, transient transfection, and viral production in suspension culture in bioreactors are a viable solution for commercial manufacturing of viral vectors (Figure 2) to meet increased demand for larger volumes of vector. Bioreactors enable improved environmental control (pH, dissolved oxygen, mixing, and temperature), true scalability, and reduced production costs. With the implementation of single-use bioreactors, the shift towards closed processing becomes a reality.

Figure 2.The suspension process for viral vector production begins with inoculating the cell bank for seed train expansion before the expanded culture is placed into a production bioreactor.
The challenges faced by viral vector manufacturers can be alleviated with development of a scalable manufacturing platform (e.g., VirusExpress® 293T lentiviral production cells). Such a platform will need to include: a chemically defined medium, suspension-adapted cells, and bioreactor process development and scale-up.
To explore viral vector platforms, see our technical articles on the development of the VirusExpress® 293 AAV production platform and the scale-up of lentivirus production using the VirusExpress® 293T lentivirus production platform.
Products
To continue reading please sign in or create an account.
Don't Have An Account?