Bioprocessing And Scale-Up Of Suspension HEK293 Cells For AAV Manufacturing
The VirusExpress® 293 AAV production platform addresses common challenges of traditional adherent cell culture by providing a scalable, suspension cell culture process using chemically defined animal component-free cell culture media. The established production process helps reduce manufacturing risk and speed to the clinic.
This technical article discusses how we:
HEK293 Clone Selection and Suspension Adaptation for AAV Production
Human embryonic kidney cells (HEK293) are the cell line of choice for research production of AAV by transient production methodology rather than cell lines that contain the SV40 large T antigen (e.g., HEK293T). This is due to the risk that while AAV packages a single stranded DNA, it could also package host cell DNA which may carry transforming genes, such as large T antigen.
Beginning with an adherent HEK293 cell line, we selected clones for high growth and high transfection capability. Afterward, several of the clones were adapted to suspension cell culture in a chemically defined medium, EX-CELL® CD HEK293 Viral Vector Media. The selected clone was chosen based upon its ability to produce high titer; the clone was manufactured and fully characterized according to GMP standards to generate a Master and Working Cell Bank at the BioReliance® facility in Rockville, MD.
AAV Production Upstream Process Development
The upstream bioreactor process development utilized a two-stage design similar to the approach used to develop the VirusExpress® Lentiviral Production Platform in which the scientists first focused on optimizing the parameters for cell growth (stage 1) and then optimized the parameters for virus production (stage 2).
Stage 1: Parameters for Cell Growth
Initial bioreactor development was performed at the bench scale in 3 L bioreactors, where several growth studies were conducted testing various bioreactor parameters, such as agitation rates and pH set points (Figure 1).
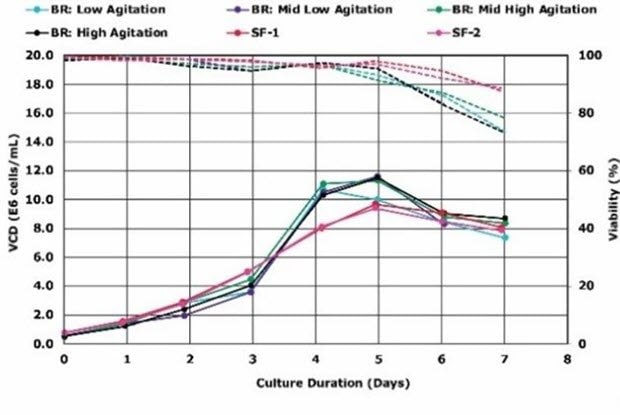
Figure 1. A)Effect of agitation rate on growth of VirusExpress® 293 AAV cells in Mobius® 3 L Bioreactors. Solid lines represent viable cell density of cells, while dashed lines represent viability. Legend: SF = 500 mL baffled flask; BR = bioreactor.

Figure 1. B)Optimization of pH for growth of VirusExpress® 293 AAV cells in Mobius® 3 L Bioreactors. Solid lines represent viable cell density of cells, while dashed lines represent viability. Legend: SF = 500 mL baffled flask; BR = bioreactor.
Once the optimal parameters for cell growth were identified, the team tested the effects of scale-up on viable cell density in a Mobius® 50 L Bioreactor. Agitation of the cells was scaled up based on power per unit volume while other parameters, such as temperature and pH, remained at the settings used at the 3 L scale (Figure 2). The growth achieved at N stage exceeded the target viable cell density (VCD) of 2x106 cells/mL for transfection.

Figure 2.Cell growth of VirusExpress® 293 AAV cells in a Mobius® 50 L Bioreactor at 10 L and 40 L working volume. The bioreactor is utilized for cell expansion (N-1 stage) prior to the virus production stage (N stage). The cell expansion starts at 10 L volume and is diluted 1:4 after three days of cell growth. Solid lines represent viable cell density of cells, while dashed lines represent viability. Legend: SF = 500 mL baffled flask; BR = bioreactor.
Stage 2: Parameters for Viral Production
After stage 1 was complete, we optimized parameters for virus production (stage 2).
Utilizing statistical analysis software and a Design of Experiments (DOE) approach, baseline upstream production parameters have been established for triple plasmid transfection with PEI transfection reagent. The upstream process flow is as follows:
- Growth (24 hours)
- Day 0: Inoculate
- Day 1: Triple plasmid transfection with PEI
- AAV2 Production (72 hours)
- Day 2-3: Monitor and sample
- Day 4: Harvest and cell lysis
The production process exceeded our target of 1x109 gc/mL by droplet digital PCR (ddPCR) for both AAV2 (9x109 gc/mL) and AAV5 (9x1010 gc/mL) (Figure 3). The robustness of the production conditions has been proven not only at the shake flask scale but also at the 3 L bench top scale. Due to tighter control of production environment in 3 L bioreactors, higher production performance is observed in the 3 L bioreactors compared to the shake flask controls.

Figure 3.AAV genome titers (ddPCR) for AAV2 and AAV5 produced in Mobius® 3 L Bioreactors (solid bars) and 250 mL shake flasks (hashed bars). Each bar represents average of replicate shake flasks or bioreactors; error bars are standard deviations.
With process improvements planned for not only transfection reagent optimization but also for cell culture media conditions, we have seen at least a 6-fold increase for AAV2 genome titers as compared to the average 3 L baseline process from 9x109 to 5x1010 gc/mL as shown in Figure 4.

Figure 4.Planned performance enhancements will achieve at least a 6-fold increase in genome titers from 9x109 gc/mL to 5x1010 gc/mL for AAV2 production.
Upstream Platform Flexibility Across Different AAV Serotypes
More than 10 natural AAV serotypes have been identified and hybrid serotypes are being engineered for AAV. The choice of serotype is critical for the therapeutic target application. The VirusExpress® 293 AAV Production Platform was used to produce different AAV serotypes demonstrating that the platform is amendable across multiple serotypes (Figure 5).

Figure 5.AAV genome titers (ddPCR) for serotype 2, 5, and 6 produced in shake flasks. Each bar represents average of replicate shake flasks and error bars are standard deviations.
For both lentivirus and AAV transient transfection production processes, the transfection complex is formed by a charge-charge interaction between the plasmid DNA and the transfection reagent, typically PEI. The transfection (PEI-DNA) complexes that are formed can change in size over time. We characterized the complex formation process and identified the narrow window of time required to generate the optimal complex size, which coincides with maximum virus productivity. From these studies, we identified that time is a critical parameter for the PEI-based transient transfection process and could be a process constraint when working with large volume bioreactors. As a result, we are exploring collaborative opportunities with transfection reagent manufacturers to determine whether there are alternatives to a PEI-based transfection reagent, specifically one that will lend itself for better scalability. Early evaluations of cationic polymer and lipid/polymer transfection agents have shown increased AAV2 production at the shake flask scale, refer to Figure 6.

Figure 6.AAV2 genome titers (ddPCR) produced using various transfection reagents in shake flasks. Each bar represents average of replicate shake flasks; error bars are standard deviations.
Products
To continue reading please sign in or create an account.
Don't Have An Account?