ELISpot Assay: Functional Cellular Immunology
What is an ELISpot Assay?
Under optimal conditions, the enzyme-linked immunosorbent spot (ELISpot) assay enables visualization of multiple secretory products from a single responding cell. Thus, the ELISpot provides both qualitative (type of immune protein) and quantitative (number of responding cells) information. By virtue of this assay’s unsurpassed sensitivity, frequency analysis of rare cell populations (e.g., antigen-specific responses) which were not possible before are now relatively easy. Recent improvements to the design of multiwell microplates, including use of membranes with reduced background fluorescence, have bolstered the widespread application of Elispot assays. When assay sensitivity, ease of use, and cost are all taken into consideration, the Elispot platform is likely the superior choice for the development of multifunctional T cell assays for the research, therapeutic, and diagnostic communities.
The immune response and the Elispot – necessity is the mother of innovation
Precise regulation of effector function is critical to mounting a potent, yet specific immune response. T lymphocytes provide the framework for this process, exquisitely orchestrating the body’s defense against infections and cancer. This is accomplished through highly selective engagement and activation of antigen-specific effector cell lineages. Depending on the strength and nature of the stimuli, a wide range of effector functions may be elicited, including cytolytic activity, secretion of multiple cytokines and other bio-active molecules, proliferation, and selective homing to sites of infection. As these T lymphocytes and their responses represent true correlates of clinical outcome, the ultimate goal in immune diagnostics has been to reliably identify that small fraction of responders, qualify their mode(s) of action, and accurately quantify the degree of response. While no one assay can measure all relevant parameters simultaneously, the ELISpot offers multi-dimensional, quantitative assessment of effector function(s) at the single cell level with superior sensitivity and resolution.
Developed in 1983,1,2 the ELISpot assay represents the convergence of plate-based enzyme-linked immunosorbent assays (ELISAs) with membrane-based Western blotting technologies, permitting detection of secreted analytes at the single cell level. Membranes offer vastly improved binding characteristics over standard polystyrene surfaces. While a number of options exist, the majority of Elispots are currently performed on polyvinylidene fluoride (PVDF) membrane plates. Binding of capture antibody (Ab) is governed by hydrophobic interactions between amino acids such as phenylalanine or leucine and PVDF; this association is much stronger than the electrostatic interactions at nitrocellulose surfaces.3,4 Stronger binding interactions translate to greater Ab density on the membrane’s surface, resulting in better-defined spots.4 Because the readout for an ELISpot is "spots/well", the PVDF membrane’s white color provides the ideal backdrop for spot detection and analysis. The microplate format further offers greater throughput and is amenable to automation; more samples, more stimuli, or greater numbers of different cytokines can be assayed simultaneously in neighboring wells.
Originally conceived for the enumeration of B cells secreting antigen-specific antibodies1,2, the ELISpot has been adapted for many tasks, the most prominent being the quantification of antigen-specific cytokine responses (Figure 1). In the standard assay, cytokine-specific Abs are immobilized on membrane-bottomed 96-well plates. Next, cells (commonly, total peripheral blood mononuclear cells (PBMCs) or purified subsets) are seeded in the presence or absence of stimulating agents. Over time, activated cells begin to secrete cytokines, which bind to the capture Ab in the immediate vicinity of the expressing cell. Cells are then washed away and spot detection is accomplished through substrate deposition following either a one-step (enzyme-conjugated, cytokine-specific Ab) or two-step (biotinylated Ab/streptavidin-enzyme) antibody binding process. Once the signals are developed, spot numbers can be tallied manually or through use of image-based spot readers with accompanying analysis software. The frequency and total number of responder cells is determined by comparing the number of spots between stimulated and untreated/control wells.
This review will highlight the unique performance characteristics, workflow attributes, and cost benefits which, when considered together, clearly identify the ELISpot assay as an excellent platform for elucidating the complexities underlying immune responses. In addition, we outline recent advances related to this technology and how these improvements provide greater benefit to the research community, whether focused on mechanistic studies, diagnostics, or therapeutic design. Lastly, the assay protocol will be discussed in detail with an emphasis on standard best practices and troubleshooting guides.
The unmatched power of the Elispot platform for T-cell functional analysis
The complexity of any given immune response is underscored by the multitude of parameters that may need to be assessed to gain clarity on the physiological mechanisms underlying the process. While many assay formats exist, those most commonly used in the study of ex vivo T cell effector function include flow cytometry, ELISpot, ELISA, multiplex bead arrays, and quantitative PCR. While all have specific strengths and limitations, Elispot assays present clear advantages, which will be highlighted in the following section.
The ELISpot , like flow cytometry-based intracellular cytokine staining (ICS), directly determine the frequency of antigen (Ag)-specific T cells, a core competency for immune diagnostics. Such resolving power is unattainable with supernatant-based assays, such as ELISAs or multiplex bead arrays, where measurements are based on bulk cytokine production by all cells in a given sample well. In acute HIV subjects, the frequency of cells producing IFNγ in response to common recall antigens (e.g., TT or PPD) was comparable to healthy donors; however, spot size is dramatically reduced5. This result suggests that HIV-specific T-cell function, and not cell number, was impaired. Similarly, T cells recently activated in vivo may show increased per cell cytokine production when compared to "e;older"e; memory T cells6,7. The ability to distinguish between long-term memory and recently activated subsets has implications for T cell diagnostics of autoimmune disorders and chronic infections. Results from bulk assays are also confounded by the contribution of background signal(s) from the innate immune system. Dilution of the Ag-specific response results in overall signal flattening; this issue is most relevant for detecting the presence of rare populations, such as circulating tumor cells (CTC) in PBMC or disseminated tumor cells in bone marrow, both early markers of metastasis8.
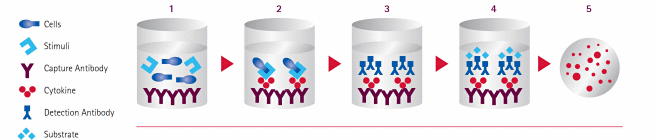
Figure 1.The ELISpot Assay Workflow. (1) Coat membrane with capture antibody. Add immune cells and stimulate. (2) Responding cells produce cytokines. The cytokine of interest binds to the capture Ab beneath the cell. (3) Wash to remove cells. Add a second cytokine-specific biotinylated Ab, which binds to the cytokine-Ab complex. (4) Add streptavidin-enzyme conjugate. (5) Add enzyme substrate and develop. Within a well, each responding cell will result in the development of one spot.
For the T cell repertoire to be capable of recognizing a potentially infinite number of infective agents while simultaneously distinguishing them from self, the total naive pool contains ≥ 1012 unique T-cell receptor (TCR) specificities. Consequently, in the absence of infection, the frequency of circulating memory cells with specificity to any one antigen is quite low, typically in the range of 1:10,000 - 1,000,0009,10. Detection of such rare events can present a significant challenge to flow-based platforms, where the lower limit of sensitivity is reported to be 0.02%11. Relative to ELISpots, the sensitivity threshold for cytokine measurements in culture supernatants is further diminished by analyte dilution in the surrounding milieu, absorption by bystander cells, and enzymatic degradation. By contrast, ELISpot assays demonstrate a detection threshold of less than 25 IFNγ-producing T cells per million PBMC (0.0025%)12,13; this equates to a near 10-fold increase in detection sensitivity. The ELISpot assay’s high sensitivity is also important for allergy research, where identifying the very low frequency Th2 cytokine-producing cells is critical for both disease monitoring and development of immune therapies14. Specifically, both flow cytometry and ELISA platforms demonstrate insufficient detection of IL-4, the predominant indicator of a Th2-driven response15.
The ELISpot is one of the few techniques permitting quantitative single cell analysis of biological function (e.g., cytokine release). With intracellular cytokine staining (ICS), where cytokine detection occurs prior to release, there is the potential for misleading results due to post-translational modulation before or during the secretory process 16 . The duration of an ICS assay is limited by the toxicity of protein transport inhibitors such as Brefeldin A or Momensin. For quantitative RT-PCR, detection is even further removed from actual function, since the target being measured is mRNA. ELISpot assays are also independent of secretion kinetics, a significant fact given the unsynchronized nature of the responding T cells pool. For ICS, all cells are killed via fixation at a pre-determined time. Cytolytic response mediators, such as granzyme B and perforin, are stored in granules then released upon proper stimuli17-19. Due to this unique regulatory mechanism, ICS will falsely identify all effector memory cells (~20% of total T cells) as perforin-positive. Perhaps of greater significance is the Lysispot assay, a modified ELISpot capable of enumerating Ag-specific cytotoxic CD8+ T cell effector function through direct target cell lysis20. Until the development of the Lysispot assay, since most cytotoxicity assays are performed on bulk cultures21, IFNγ ELISpots were commonly used as correlates of CD8+ cellular immunity22-24.Use of the Lysispot in the study of HIV revealed that not all IFNγ producing cells were capable of killing25. This finding also highlights the need for greater multiplicity of detection in single cell immunoassays.
T cells occur in a wide range of effector classes, and expression of one or more of these can vary greatly depending on the type of pathogen and the subject’s immune status. Cumulative findings in the area of tuberculosis (TB) diagnostics suggest that differences in the cytokine signature may provide a clearer distinction between asymptomatic latent and active forms of the infection. The rapid identification of active cases is most critical as these individuals pose the greatest health risk to the community26-30. While bead-based quantitation in supernatants offers multiparameter analysis, it suffers from limitations precluding acceptance as a diagnostic platform for TB and other diseases. By contrast, ELISpots are amenable to multiplex analyses carried out simultaneously (single well) or in parallel. Well-established dual-color ELISpots, using both enzymatic and fluorescent approaches, are currently used in many research settings. Fluorescent ELISpots, or FluoroSpots, offer significant advantages over colorimetric formats, particularly in the areas of multiplexing and automated spot detection. Moreover, as spot development is not enzymatic, signal intensity is directly proportional to the amount of analyte within the spot and therefore far more quantitative.
Increasing the multiplexing capacity beyond two colors requires membrane surfaces with minimal fluorescent background signal. Due to their highly porous nature, membrane surfaces are very rough.
For this reason, they scatter light and exhibit high fluorescence background. While PVDF membrane (Immobilon®-P membrane) is purported to be a better surface than nitrocellulose for FluoroSpots, the Immobilon®-FL PVDF membrane variant was designed specifically for fluorescence detection in Western blotting applications and exhibits background fluorescence signal that is nearly 1/100 that of standard PVDF. Data showing the use of Immobilon®-FL membrane in two-color IFNγ/IL-2 FluoroSpots is presented in Figure 2 (images) and Figure 5 (page 6, spot counts). Beyond multiplexing, FluoroSpots permit distinction of two simultaneously measured functional outputs. Multiplexing also serves to reduce required sample size. The ever-expanding availability of discrete fluorochromes, when combined with multi-fluorescent imaging instrumentation and fully automated sample acquisition and data analysis, provides the framework for unsurpassed polyfunctional analysis of Ag-specific T cell responses via ELISpots.
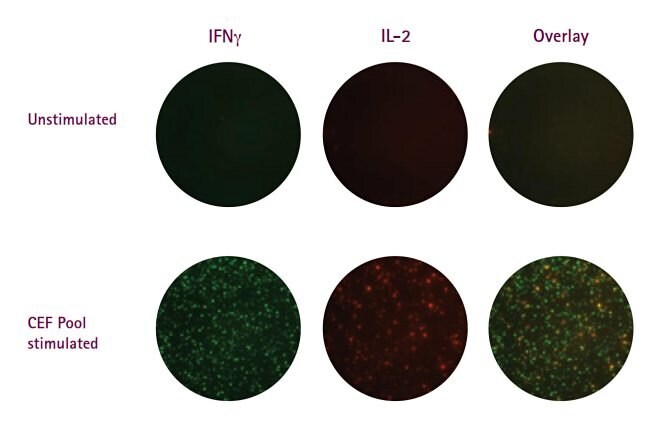
Figure 2.Representative images from two-color IFNγ/IL-2 FluoroSpot assays performed on the Multiscreen® HTS plates fitted with FluoroSpot-optimized Immobilon®-FL PVDF membrane. CEF pool refers to pool of peptides covering epitopes of Cytomegalovirus, Epstein-Barr virus and flu virus.
Beyond the membrane and plate material, plate color can also greatly impact the success of the FluoroSpot. The data presented in Figure 3 highlights the differences in image quality between different Multiscreen®HTS plate formats. IFNγ/IL-2 FluoroSpots were performed on PBMC cells following culture (250K/well) in the presence of CEF peptides. From a strictly visual perspective, spot clarity was roughly equivalent on the clear and black formats (Figure 3A). By contrast, white plates showed high background signal, making spot detection difficult, particularly in the Green channel (IFNγ-FITC). The high background occurred even after a significant reduction in exposure time (roughly 1/5). High background was most likely due to increased reflectance as compared to black or clear frames where light is either absorbed by or passes through the surrounding plate material, respectively. A comparison of spot counts demonstrated a significant reduction in "spots counted" on white plates when compared to either black or clear formats (Figure 3B-C). Once again, the discrepancy was more significant for IFNγ spots, where almost 80% reduction in total spots was observed. The background issue may be eliminated if wells were punched out and analyzed separately; however, this may not be practical if large experiments are to be performed. Given their similarities in performance, the clear plate format offers the more practical option, as it also facilitates visually monitoring reagent addition. It should be noted that all analyses were performed using the iSpot™ system (AID). Due to differences in performance characteristics, other fluorescent plate readers, may not demonstrate the same plate preference.
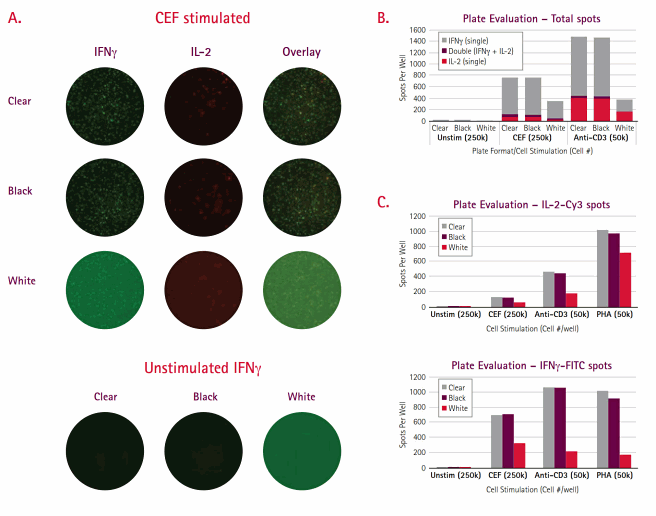
Figure 3.Shown are representative well images for dual color IFNγ/IL-2 FluoroSpot performed on total PBMC in three different Multiscreen®HTS plate formats (Clear, Black, and White) using Mabtech’s FluoroSpot kits. For CEF-stimulated wells, displayed are images for each individual cytokine as well as the overlay image. For unstimulated wells, only the IFNγ single color data are shown. (B) The graph presents summation data for each plate format across three culture conditions. Each bar is segregated into three parts – IFNγ, IL-2, and dual responder spots. All bars represent the average of 3 replicates. (C) The two bar graphs show comparative spot data for each cytokine measured. All bars represent the average of 3 replicates.
Unlike flow cytometry, where instrument priming can result in sample loss, every cell in an ELISpot is measured. The ELISpot also, on average, requires one-tenth as many cells per test, which provides a crucial advantage under conditions where samples are precious (remote settings) and/or limiting (pediatric or immunosuppressed test subjects). One long-standing problem with the 96-well microplates has been the waste of unused wells in small-scale assays such as that occurring in diagnostic analysis of a single patient sample. We offer 8-well strips (Product No. M8IPS4510) designed for the diagnostic community; this format is particularly attractive to resource-limited countries where diseases such as TB and HIV are most devastating (Figure 4A). 28 Constructed in a transparent format, the strips are suitable for FluoroSpots, as well as standard enzymatic options, and perform comparably to the standard 96-well plate (Figure 4B, 5). The 8-well strips are currently part of Oxford Immunotech’s T-SPOT.TB Test, an FDA-approved IFNγ ELISpot test designed specifically for diagnosis of tuberculosis infection.
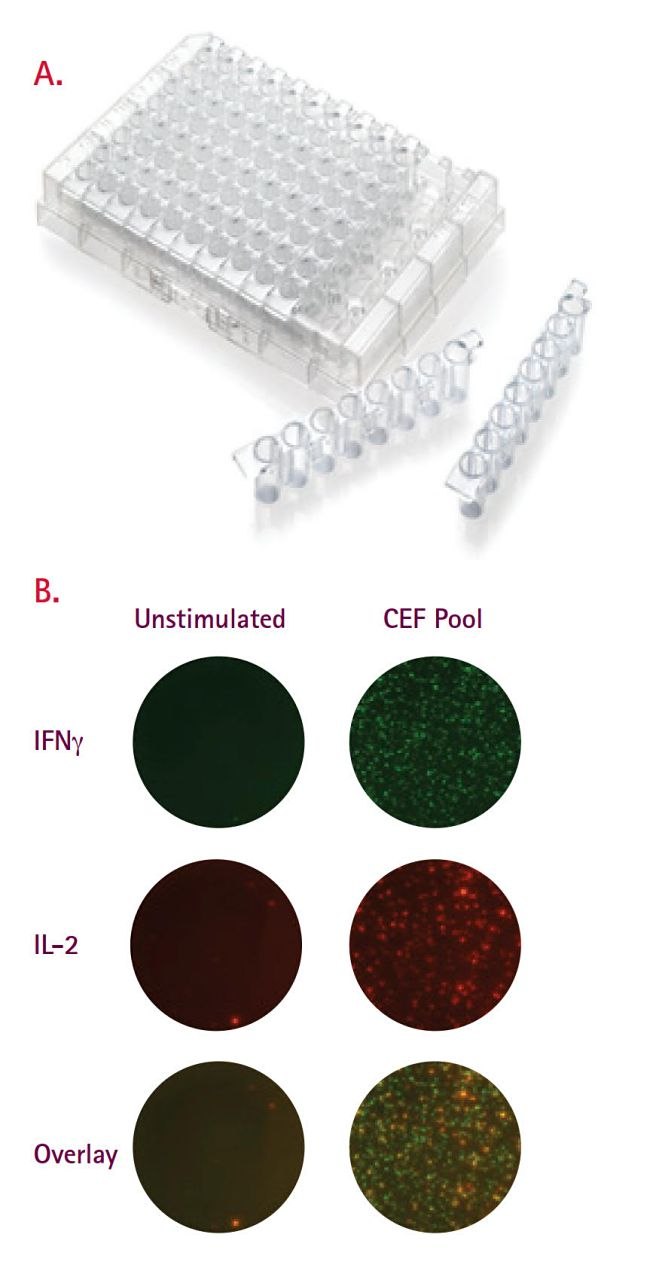
Figure 4.(A) Membrane-bottom, 8-well strip plate designed specifically for diagnostic ELISpot. This format is part of T-SPOT.TB (Oxford Immunotech), a commercially available IFNγ ELISpot kit designed specifically as a diagnostic for tuberculosis infection. (B) Representative images (single and overlay) from two-color FluoroSpot performed in 8-well strips. IFNγ/IL-2 FluoroSpots were performed on healthy untreated PBMCs left untreated and following stimulation with CEF peptides. All assays were performed using FluoroSpot kits (Mabtech).
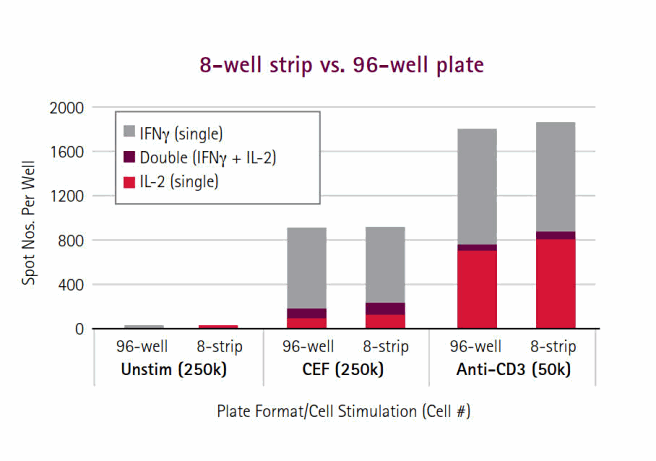
Figure 5.8-well strips perform similarly to the standard 96-well MultiScreen® plates. The bar graph presents summation data for each format across three culture conditions. Each bar is segregated into three parts – IFNγ, IL-2, and dual responder spots. All bars represent the average of 3 replicates. Working with far fewer cells per assay also means that multiple replicates can be performed, thereby increasing statistical power. Such discriminatory capacity is not possible with bulk assays. Although ELISpot and flow cytometry assays have similar protocol steps, ELISpot data acquisition/analysis is far easier to perform and less time-consuming than flow cytometry. In fact, data from a 96-well ELISpot plate can be acquired and analyzed by an image-based platform as rapidly as it takes a skilled flow cytometer operator to analyze one sample containing 300,000 cells. For certain cytokines, the signal:noise ratios for ICS are low and often non-bimodally distributed, making gating decisions arbitrary and difficult. While flow cytometry struggles with a lack of user-independent gating algorithms for sub-population analysis, and therefore suffers from subjectivity and lab-to- lab variation, automated platforms promote the standardization of ELISpot data analysis and greater reproducibility across sites31. This combination of features also makes ELISpot the ideal choice for high-throughput testing applications, which could be applied in large-scale subject profiling. For example, IFNγ ELISpots are commonly used as a correlate of vaccine efficacy to identify potential candidates for HIV and other diseases.32-33
Assay miniaturization can simultaneously reduce cell requirements while increasing throughput. The data presented in Figure 6 is part of ongoing studies performed at Cellular Technology Limited (CTL) to validate the application of ELISpots to a 384-well format.34 In this example, IFNγ ELISpots were performed on PBMCs following stimulation with CEF-7 peptide. Plates were imaged and analyzed using CTL’s ImmunoSpot® S6 Micro Analyzer. For the range of seeding densities tested, the assay demonstrated a strong linear relationship (R2=0.9866) between spot-forming units (SFU) and cell number (Figure 6B). Lastly, modifications to microplate design have increased compatibility with existing robotics systems, thereby also improving potential throughput. These plate adaptations include stricter dimensional specifications and rigid side walls. Plates are now fully compatible with standard fluidics platforms, plate washers, and devices for imaging and image analysis.35
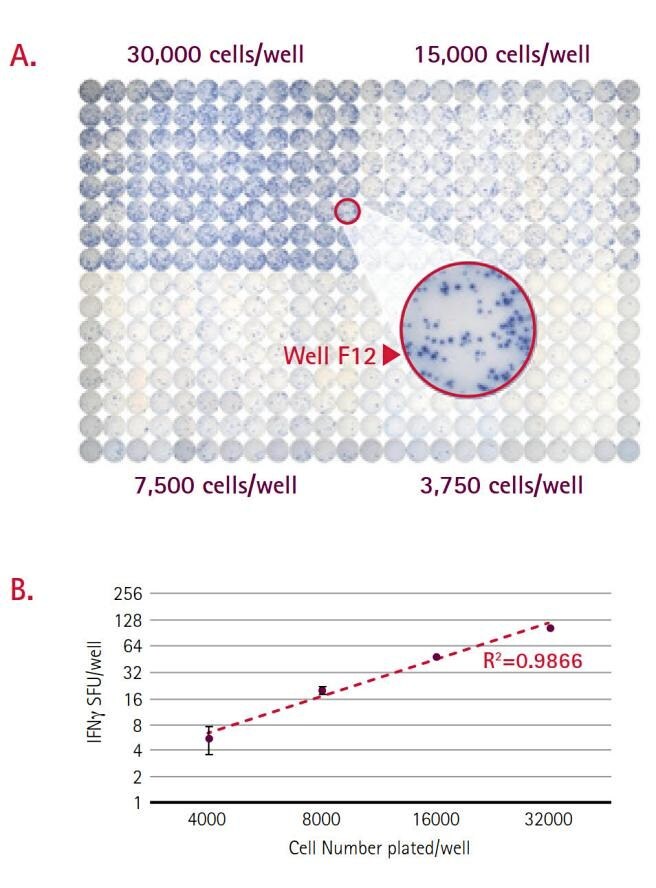
Figure 6.(A) A representative image from an IFNγ ELISpot performed in 384-well plates on four seeding densities of PBMC (n=96 per level). Well F12 has been magnified to demonstrate spot clarity. (B) In this graph, IFNγ spot forming units (SFU) were plotted against seeding density. Each data point represents the average of 96 replicates and error bars represent standard deviation. The assay shows a strong linear response for range of cell quantities tested.
Elispot Optimization
While ELISpot assays permit frequency determination for very rare events, data interpretation can become ambiguous when (1) spot numbers in antigen-containing wells are low, (2) spot counts in negative control wells are elevated, and particularly when both occur simultaneously. Thus, the primary task, even before statistics are employed, must be the optimization of basic assay parameters and reagents to maximize the signal-to- noise ratio. While the use of highly specific ELISpot–validated Ab pairs is key to assay success, proper consideration and execution of a number of other steps are required to ensure optimal performance. This section will provide an overview of the standard T cell ELISpot assay with particular emphasis placed on the experimental rationale behind underlying pivotal steps and suggestions for troubleshooting erroneous or ambiguous results.
Initial Thoughts
Choice of Plate (Membrane)
PVDF membrane plates (Catalogue Nos. MSIPS4W10, MSIPS4510, MAIPSWU10, MAIPS4510) are recommended, over a mixed cellulose ester format (Catalogue No. MSHAS4510), due to slightly improved binding of capture Ab and superior performance in spot detection, particularly for fluorescent applications. The one drawback of PVDF plates is the extreme hydrophobicity of the material,33 a property that may necessitate pre-wetting with alcohol prior to addition of the coating Ab. The potential pitfalls of this step are outlined in a later section. Since the mixed cellulose membrane is hydrophilic, ELISpots can be performed without pre-wetting,36,37
Negative/Positive Controls
Relevant controls are crucial to measuring Ag-specific responses via ELISpot. Negative controls routinely consist of cells cultured without stimuli, whereas polyclonal T-cell activators are commonly used as positive controls to confirm both cell and assay functionality. Positive controls include anti-CD3/CD28 Abs, phytohemagglutinin (PHA) and concanavalin A (ConA). These activators induce secretion of many common cytokines including IFNγ, IL-2 (Th1), IL-4, IL-5, IL-10 and IL-13 (Th2). Another common control is the commercially available CEF (Cytomegalovirus, Epstein-Barr virus, Influenza virus) peptide pools. These consist of multiple epitopes from each of the three viruses, to which most healthy individuals (~ 90%) possess CD8-responding T-cells.38
Plate Organization - Edge Effects
The plate is an array of 8 rows with 12 wells in each. Wells at the periphery of the plate (columns 1 and 12, Rows A and H) are in greater direct contact with surrounding environment and thus may differ from interior wells. Specifically, medium evaporation from peripheral wells in prolonged cultures may impact overall assay performance. Where possible, the use of "media only" wells around the periphery of the true sample wells can minimize this effect.
The Question of Pre-Wetting
Comparative testing has previously demonstrated that proper ethanol pre-treatment of PVDF-based MultiScreen®HTS plates (15 µL of freshly prepared 35% v/v ethanol followed immediately by water washes) can lead to increased spot number (better sensitivity) and more sharply defined spots (for more accurate quantitation) (Figure 7)35. That said, pre-wetting is not universally applicable to all ELISpots ; its requirement is dependent on the inherent hydrophobicity of the capture Ab; therefore, the pre-wetting protocol should be optimized prior to application35,36. Overtreatment with larger volumes of alcohol, longer exposure time, or more concentrated alcohol can lead to trapping of residual liquid between the membrane and underdrain, which may result in poor assay performance or, more critically, well leakage. Leakage associated with alcohol pre-wetting is not a concern when using ELISpot plates lacking an underdrain (Catalogue No. MAIPSWU10); however, this format may suffer from potential media evaporation during extended culturing as well as sterility issues surrounding the exposed base membrane. Another alternative is to use microplates made with hydrophilic membrane such as mixed cellulose ester. It is also important to note that once plates are ethanol-treated, they must be kept wet for the entire assay.
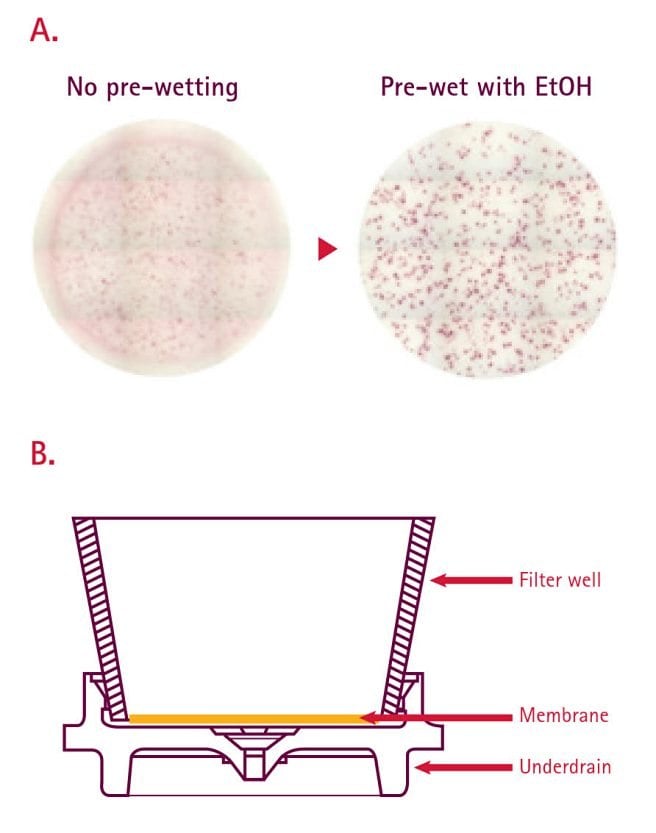
Figure 7.(A) Representative well images for mouse IFN gamma ELISpot performed on PVDF membrane-bottom plates with and without ethanol pre-wetting. (B) A schematic showing the architecture of an individual well in a MultiScreen®HTS plate. Recent design changes to increase the spacing between the base of the membrane and top of the underdrain have reduced the incidence of leaking.
Coating with Capture Antibody
In traditional ELISAs, binding to the surface occurs via passive adsorption and requires alkaline conditions (0.2 M sodium carbonate/bicarbonate pH>9) to maximize the electrostatic component of the protein:polystyrene interaction. By contrast, coating of PVDF membranes for ELISpot is mediated solely by hydrophobic forces. For this reason, a phosphate-buffered saline (PBS) buffer (pH 7.4) is commonly used. Membranes also offer significantly greater surface area (300X) for binding than do polystyrene plates39. To ensure performance while maximizing cost efficiency, it is critical to standardize the amount of capture Ab used per well. For optimal performance, we recommend initial titration of both the coating and detection Abs in tandem. Typically, a good starting point for ELISpot coating is 0.5-1 µg Ab per well (5-10 µ/mLin 100 µL); this is 5-10X greater input than for ELISAs. Lower input can result in more diffuse spot morphology as well as reduced spot number. Both parameters need to be considered when validating new assay protocols, particularly when determining quantitative expression (spot size) or low frequency events, respectively. Mabtech offers a wide range of fully validated ELISpot Ab pairs (coating and detection) for the assay of human samples as well as other species (For details, see www.mabtech.com).
Blocking Step
Following incubation with capture Ab, plates should be washed extensively, then blocked and equilibrated for 2 hours at 37 °C with the same culture medium (200 µL/well) that will be used during cell stimulation (minus activator). Once in blocking medium, sealed PVDF plates can be stored overnight at 4 °C. Longer storage can result in protein precipitation and reduced spot resolution. It is important to note that when plates are removed from 4 °C and allowed to reach room temperature, the sealing tape must also be removed to prevent leakage due to gas expansion.
Cells - Plating and Stimulation
Fresh vs. Frozen
PBMCs or enriched T-cell subsets constitute the bulk of ELISpot assays. Once purified from blood, PBMC can be cryopreserved and thawed without loss of functional activity40. Given the often precious nature of disease samples, there are multiple benefits to cryopreservation: (1) data can be independently reproduced, (2) multiple analytes can be assessed, (3) patient samples can be stockpiled and assayed simultaneously, thus minimizing the potential inter-assay variability. For optimal recovery of viable cells, follow these recommendations: (A) during freezing, have cells and freezing medium at room temperature prior to mixing and (B) during thawing, to minimize osmotic lysis during washing of thawed cells, transfer cells to a 15 mL conical tube on ice and slowly add cold medium.
The Benefit of Benzonase® Nuclease
An additional challenge with using frozen PBMCs is cell clumping during the thawing process. Clumping is often caused by the presence of free DNA and cell debris; it appears to be related to both the donor source and blood handling. In particular, clumping occurs more frequently when blood has been stored overnight prior to PBMC isolation. Spot count results for overnight-stored blood showed a dramatic decrease when compared with responses for the corresponding PBMCs isolated from fresh blood.36 The greatest decreases in signal were detected for samples in which the highest degree of cell clumping was observed. To improve assay performance, we recommend addition of Benzonase® nuclease (Product No. 71205), which degrades all forms of DNA and RNA, to the assay medium for the first two wash steps during the thawing procedure. The results from overnight blood PBMCs processed with Benzonase® nuclease more closely approximate the results obtained with cells isolated from fresh blood. Moreover, Benzonase® nuclease addition resulted in no changes to cell viability or changes in the expression of certain surface markers, including CD4, CD8, CD38, or CD62L.36
Cell Counting and the Value of Percent Viability
Once Ab steps have been standardized, differences in quantified cell yield and integrity of each sample presents the greatest source of assay variability. While total cell counts are important, a more critical factor to consider when setting up the culturing component is cell viability. Determining the percentage of dead and apoptotic cells is not only important for culture setup, it also provides quantitative information on the overall quality of the sample. The latter component is particularly useful when assessing success/failure of the freeze-thaw process. Manual counting methods, such as Trypan Blue exclusion using a hemacytometer, lack accuracy due to user subjectivity. Further, these methods do not provide a measurement of the apoptotic fraction. Automated cell counting via flow cytometry using fluorescent dyes, such as the Muse™ cell analyzer and ViaCount® reagent, demonstrated superior precision to manual methods for the enumeration of viable cells.41
Cells Per Well and Replicates
On average, T-cell ELISpot counts show linearity for PBMCs in the range of 100,000-800,000 cells5,9-13. Where possible, cells should be serially diluted and plated in triplicate. Unfortunately, given the restrictions of well size in 96-well plates (0.3 cm2), seeding more than 400,000 cells per well may result in overcrowding and cell stacking. The consequence here is creation of diffuse spots due to indirect contact of the cells with the Ab-coated membrane. To best monitor instances where the frequency of Ag-specific responders is low, and higher cell loads are required, either perform assays in larger wells or perform replicate wells at maximal cell density. By using replicate wells, spot counts from all the wells can be summed to derive the response frequency (SFU/total cells seeded).
During incubation, we do not recommend plate-stacking, as this can lead to variations in temperature between the plates and potentially differences in spot size and/or number. Also, it is important that plates are subject to minimal agitation, because movement can lead to localized cytokine diffusion and loss of spot sharpness.
Detection - Chromogenic vs. Fluorescent options
Following stimulation, cells are removed, wells extensively washed, and a second analyte-specific Ab is applied. At this and all subsequent steps, washing is critical for the complete removal of cells, nonspecifically bound Ab, and detection reagent. Incomplete removal of unbound reagents will lead to an overall increase in background signal. Potential challenges surrounding washing and spot development will be addressed in the troubleshooting section (page 10).
ELISpot assays may be performed either with antibodies directly conjugated to the detection motif (enzyme or fluorochrome) or as a two-step process involving a biotin/streptavidin-conjugated Ab pair. While the two-step process offers greater intensity due to signal amplification, and therefore may be preferable in cases where cytokine production per cell is low (allergy/Th2 responses), this protocol also suffers from a greater potential for background staining due to nonspecific interaction with the coating Ab. With enzymes, such as horseradish peroxidase (HRP), a precipitating substrate (TMB or AEC) is used for spot detection. Due to HRP’s high turnover rate, spot development is fast (≤5 minutes). By contrast, spot development using alkaline phosphatase-conjugated Abs is far slower but with apprecibly lower background. For chromogenic assays performed on MultiScreen®HTS plates (those with underdrains), it is recommended that the underdrain be removed before substrate addition; failure to do so can result in high background staining. Once removed, plates should be propped up to minimize membrane contact. To enhance spot visualization, plates should be dried without a lid, upside down, at room temperature for several hours. For long-term storage, plates should be kept in a dark, dry place at room temperature to prevent bleaching of spots.
As previously discussed, the use of fluorescent conjugates offers significant advantages over colorimetric schemes especially for dual cytokine applications or where greater quantitative assessments of individual spots is desired. While FITC- and Cy3-conjugated Abs are commonly used, the choice of fluorescent probe is limited only by the availability of conjugates and detection platforms.
Spot Counting and Analysis – What is a real spot?
Each spot represents the ‘cytokine signature’ of a single cell. Due to diffusion properties, a true spot has a densely colored center which fades toward the edges; the size and/or color intensity of the spots is determined by the amount of cytokine released. That said, due to differences in analyte measured, incubation time, antibody concentration, enzyme activity, substrates and other materials used as well as the functional state of the cytokine-secreting cells, spot size and density can vary greatly. Artifactual spots may appear and can be caused by the aggregation of antibodies or the incomplete removal of cells and cellular debris. Morphologically, these spots can be differentiated from ‘true’ spots by their homogeneity in color intensity and sharper (non-rounded) edges.
From the above description, manual spot counting by light microscopy would be classified as a highly subjective process, fraught with a great degree of inter-user variability. Further, when considering the sheer number of wells that may need to quantified in a standard vaccine trials, the task of ELISpot data analysis becomes a far too laborious task for human eyes. The availability of sophisticated ELISpot readers offers a complete solution for precise evaluation of spot data. These instruments include features to overcome problems with variable background intensity and the ability to distinguish true single cell spots from artifacts. The latter capability relies upon the use of minimum and maximum threshold values for spot size and intensity, permitting the exclusion of weak bystander responses and clusters containing multiple cells, respectively. Beyond speed, spot analysis software offers process standardization, a critical component when studies are performed across sites, such as is the case for diagnostic testing and vaccine trials. Moreover, ELISpot readers and analysis software open the door for more precise measurements of spots, permitting the quantitation of secretion of multiple cytokines on a per-cell basis.
A Troubleshooting Guide for Interpreting and Correcting Ambiguous Elispot Result
1. High Levels of Background Staining
2. No Spots/Blank Wells
3. Fuzzy/Poorly Defined/Confluent Spots
Acknowledgements
We would like to thank Tomas Ernemar (Mabtech) for providing all the FluoroSpot data as well as manuscript review. The 384-well data was kindly provided by Jodie Hansen (CTL). We would also like to thank Sylvia Janetzki (ZellNet Consulting) for manuscript review.
Products
References
To continue reading please sign in or create an account.
Don't Have An Account?