Enzyme Inhibition By Reaction Conditions
Changing certain reaction conditions can unintentionally lead to the inhibition of enzymes in your experiments. Learn how to classify enzyme inhibitors and uncover more about enzyme inhibition by reaction conditions including the effects of changing the reaction temperature and pH, as well as tips on how to properly measure pH.
Read more about
Classification of Enzyme Inhibitors
Enzyme inhibitor classification depends on a variety of factors that affect enzyme activity. By their catalytic nature, enzymes accelerate the rate of a reaction without getting altered by their participation in the reaction. The ability of an enzyme to catalyze a reaction can be reduced by binding various small molecules (inhibitors) to the active site or, sometimes, at a site away from the active site.
Enzymatic activity depends on several factors. The most important factors that affect enzyme activity are:
- Enzyme concentration
- Amount of specific enzyme substrate
- pH of the reaction medium
- Temperature
- Presence of activators and inhibitors
Enzyme inhibitors are usually low molecular weight compounds that combine with the enzyme to form an enzyme-inhibitor complex, either reducing or completely inhibiting the catalytic activity of the enzyme and therefore reducing the rate of reaction. The binding of an inhibitor to the active site of the enzyme can block the entry of substrate to the site. Alternatively, some inhibitors can bind to a site other than the active site and induce a conformational change that prevents the entry of substrate to the active site. Based on the type of interaction with the enzyme, inhibitor binding can be classified as either reversible or irreversible (Figure 1).
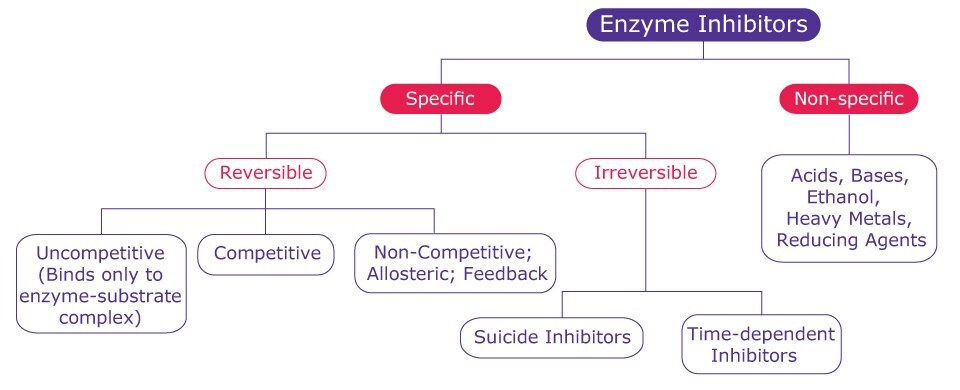
Figure 1.A general classification of enzyme inhibitors.
Inhibition By Temperature Change
Most enzymes are stable over a wide range of temperatures. However, they work best in the physiological range because temperature changes can cause inhibition. Enzymatic activity can be reduced significantly by lowering the reaction temperature and can be increased by increasing the reaction temperature, but only up to a certain limit. Since enzymes are proteins, they are partially unfolded or denatured at higher temperatures (Figure 2). Hence, the reaction can also be terminated by bringing the reaction mixture to a high, denaturing temperature.
Effect of Increased Reaction Temperature
When the reaction temperature is increased, the rate of the reaction increases, based on the principle of Q10. The Q10, or temperature coefficient, is a measure of the rate of change of a biological or chemical system as a consequence of increasing the temperature by 10°C. In biological systems, the rule of thumb is that for every 10°C rise in temperature, the rate of the reaction doubles. However, when the temperature becomes too high, proteins are denatured, the enzymatic activity is lost, and the organism will perish. Q10 can be calculated by the following equation, where R1 and R2 are reaction rates at temperatures T1 and T2, respectively.
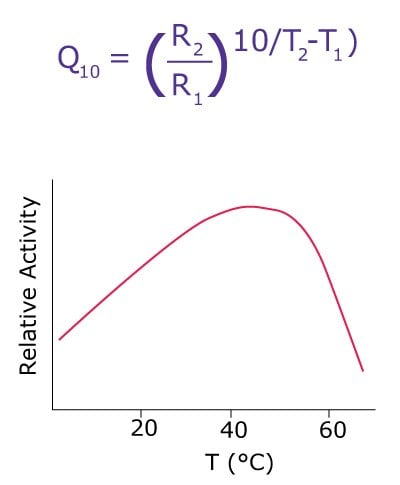
Figure 2.Effect of temperature on enzyme reactions.
Effect of Decreased Reaction Temperature
Similarly, decreasing the reaction temperature can reduce enzyme activity, because low temperatures can change the shape of the enzyme. However, in most cases, when the temperature is brought up to the physiological range, enzyme activity is restored.
Effect of Freezing and Thawing
It is important to note, however, that enzymes are sensitive to repeated freezing and thawing. Freezing can induce several stresses such as:
- Ice formation
- Changes in solute concentration due to the crystallization of water
- Eutectic crystallization of buffer solutes
- Resulting changes in pH
Hence, it is best to thaw a frozen enzyme only once and then aliquot into single-use vials before refreezing.
Tips for Changing the Reaction Temperature
A general tip for changing the reaction temperature is to bring the incubation medium to the desired temperature (for example, 37°C) before adding an enzyme or inhibitor. Also, if the enzyme reaction is to be terminated by increasing reaction temperature to the denaturing point, it is best to immerse the reaction tube in a boiling water bath instead of gradually raising the temperature. This is particularly important in experiments with shorter incubation periods.
Inhibition By pH Change
pH has a clear effect on rates of enzyme-catalyzed reactions and pH changes can also lead to enzyme inhibition. Each enzyme has a pH optimum above or below which its activity declines or is completely abolished.
Enzymes are active only in a narrow pH range due to:
- pH sensitivity of substrate binding
- Reduced catalytic efficiency of the enzyme
- Ionization of substrate
- Protein structural changes (usually at pH extremes)
Amino acid side chains act as weak acids and bases that perform critical functions in the active site of enzymes. Hence, any change in their ionization state can adversely affect enzyme activity. The pH range over which enzyme activity changes can provide important information about which amino acids are involved in organizing the active site. For example, a change in enzyme activity near pH 7.0 indicates the presence of histidine residue(s) at the active site.
Because enzymes are sensitive to pH changes, most living systems have highly evolved buffering systems to maintain an intracellular pH. Although most mammalian cells maintain a pH around 7.2, within intracellular compartments or certain organs, pH can be vastly different (Figure 3). For example, in the stomach, the pH is usually between 1 and 2, which is optimum for pepsin activity. Pepsin activity is rapidly lost when pH is increased to 4 or above. On the other hand, pH in the intestine is slightly alkaline, which is required for optimum chymotrypsin activity. Bicarbonate released from the pancreas contributes to this alkalinity and also neutralizes acidified food entering the duodenum from the stomach. Additionally, in cells, lysosomal compartments have acidic pH to provide optimum conditions for acid hydrolases, which lose their activity if released into the cytosolic compartment.
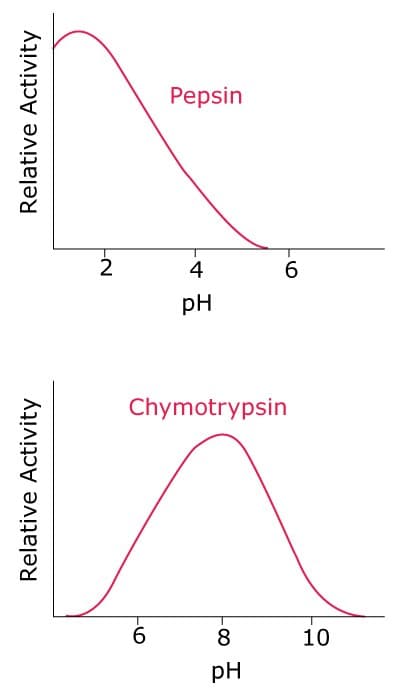
Figure 3.Effect of pH on the activity of different enzymes that are active in different organs.
Useful Tips on pH Measurements
Proper technique is critical to accurate pH results. Here are some useful tips to follow when measuring pH:
- A pH meter may require a warm-up time of several minutes. When a pH meter is routinely used in the laboratory, it is better to leave it “ON” with the function switch at “standby.”
- Set the temperature control knob to the temperature of your buffer solution. Always warm or cool your buffer to the desired temperature before checking the final pH.
- Before you begin, make sure the electrode is well-rinsed with deionized water (DI water) and wiped off with a clean absorbent paper.
- Always rinse and wipe the electrode when switching from one solution to another.
- Calibrate your pH meter by using at least two standard buffer solutions.
- Do not allow the electrode to touch the sides or bottom of your container. When using a magnetic bar to stir the solution, make sure the electrode tip is high enough to prevent any damage.
- Do not stir the solution while taking the reading.
- Inspect your electrode periodically. The liquid level should be maintained as per the specification provided with the instrument.
- Glass electrodes should not be left immersed in solution any longer than necessary. This is important, especially when using a solution containing proteins. After several pH measurements of solutions containing proteins, rinse the electrode in a mild alkali solution and then wash it several times with DI water.
- Water used for the preparation of buffers should be of the highest possible purity. Water obtained by a method combining deionization and distillation or reverse osmosis is highly recommended.
- To avoid any contamination, do not store water for longer than necessary. Store water in tightly sealed containers to minimize the number of dissolved gases.
- One may sterile-filter the buffer solution to prevent any bacterial or fungal growth. This is important when large quantities of buffers are prepared and stored over a long period of time.
Learn more about enzyme inhibition by finding answers to common questions in our FAQs on preparing inhibitors.
To continue reading please sign in or create an account.
Don't Have An Account?