Micro- and Nanofabricated Biomaterials
Introduction
Biomaterials science involves the design and fabrication of smart materials for studying, directing, or mimicking biology. For successful integration of biomaterials in biological research, a meaningful understanding of biological systems is required. Furthermore, since we are at the interface of materials science and biology, advancements in the latter are often sparked by breakthroughs in the former. Accordingly, the progress in micro- and nanotechnology has significantly enhanced our understanding of cells and microtissues. Here, we discuss interesting advances in biomaterials in the context of cellular and multicellular systems.
At the Cellular Level: Fluorescent and Inorganic Probes
At the cellular level, fluorescent probes are one of the most commonly used tools for visualizing and quantifying biological events and signals. Fluorescent protein-based indicators can target subcellular compartments and be incorporated into a wider variety of tissues and intact organisms than simple organic dyes. In addition, fluorescent probes cause less photodynamic toxicity than organic dyes while still maintaining a high temporal and spatial resolution. A multitude of fluorescent probes are derived from the jellyfish Aequorea victoria (AFPs) including green fluorescent protein (GFP) (Product No. 11814524001), red fluorescent protein (RFP), and cyan fluorescent protein (CFP), to name just a few. The engineering of these probes provides tools to investigate multiple complex processes within live cells. However, rapid photobleaching and high sensitivity to chloride fluctuations and pH have driven the development of more stable AFP variants.1 One example is coral green fluorescent protein (CGFP) which, despite its lack of brightness and broad emission and excitation peaks, is highly pH-resistant, making it ideal for use in labeling acidic cellular organelles. AFPs have been especially valuable as Fluorescence Resonance Energy Transfer (FRET)- based indicators. FRET occurs when two fluorophores are in proximity of each other (80 Å) and the emission spectrum of the donor overlaps the excitation of the acceptor. The ratio of acceptor to donor fluorescence is used as a readout for measuring biochemical events in cells. Fluorescent probes are continually being improved to provide new insights into the biology of live cells. For example, a new generation of highly sensitive near infrared fluorescent probes may be able to detect subtle conformational changes in proteins, thereby improving detection limits and applicability of AFP-based reporters.2 Additionally, single-cell and single-molecule imaging can elucidate cell-to-cell variability and permits the visualization of individual molecular interactions, both of which are challenging for existing techniques.
As an alternative to fluorescent-based probes, inorganic nanoparticles, such as gold nanoparticles, iron oxide nanoparticles, and quantum dots (QDs) can be synthesized in different sizes, shapes, and yields by varying reaction times and solvents. Methods to synthesize inorganic nanoparticles include microemulsions, thermal decomposition, hydrothermal and solvothermal synthesis.3 Collectively, these particles have been used in a variety of biomedical applications including fluorescence and magnetic resonance imaging, cell targeting, and drug delivery.3 In a study on intestinal absorption, Simovic et al. characterized the physicochemical properties and in vivo drug delivery capabilities of nanoparticles consisting of a hydrophobic phospholipid core and a PEG hydrophilic corona (Figure 1). These PEGylated phospholipid nanoparticles can solubilize and retain water-insoluble anticancer drugs while maintaining a size distribution close to the drug-free particles.4 Of particular note are QDs, which have recently permeated methods in cellular and molecular biology. Composed of a semiconductor core (Cd or Se) with a shell (ZnS), the optical characteristics of QDs are drastically improved over organic fluorescent dyes. Tunable core sizes of QDs generate a wide range of narrow emission peaks and broad excitation spectra. These characteristics make QDs ideal for single-molecule tracking (SPT), fluorescence multiplexing, and FRET. The use of quantum dots in SPT has even led to new insights into the dynamics of cell surface receptors. For example, water stabilized QDs have been used to track receptor-mediated endocytic trafficking events in live cells using fluorescence microscopy.5 The use of QDs as FRET donors, however, is still an emerging technology. Advancements in the generation of brighter and smaller QDs will allow the development of combined SPT and FRET techniques, revolutionizing the field of membrane receptor dynamics, activation, and transport.5
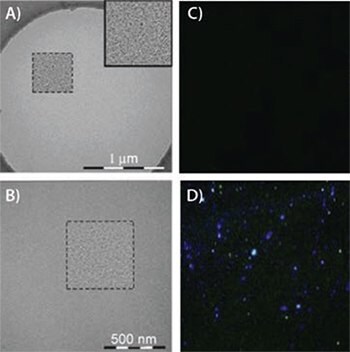
Figure 1.Transmission electron microgram (TEM) image of PEG nanoparticles (A). Higher resolution was accomplished by band pass filtering (B). Disulfide cleaved PEG2000-SS-Biotin nanoparticles do not fluoresce with the addition of avidin-FITC (C). However, intact PEG2000-SSBiotin particles bind avidin-FITC and are visualized via fluorescence microscopy (D). Adapted from Reference 3 with permission.
At the Cellular Level: Cellular Interactions
Beyond imaging, the advancements of smart materials and sensors facilitate the study of the physical forces between cells and their environment. Quantifying the forces involved in cell–cell and cell–matrix interactions has led to a greater understanding of the feedback of external-to-internal physical cues and their role in affecting cell behavior. Initially, Harris et al. developed a system of culturing cells on soft silicone substrates to study cell locomotion and its associated forces on the surrounding matrix.6 The soft substrate permits any forces exerted by the cells on the silicone to be visualized in the form of wrinkle formation on the substrate surface. Using a combination of microneedles and counterweights to re-create these wrinkles, shear forces exerted on these surfaces can be calculated.6
Technology for studying cellular forces is much more sophisticated today. Various forms of force microscopy now exist, allowing for a more quantitative measurement of the forces exerted by cells, either on one another or on the matrix around them. Traction Force Microscopy (TFM) is an evolution of the system developed by Harris et al. Advancements in TFM, such as the ability to analyze more than two dimensions, has opened up the possibility of studying not just the traditional shear traction in the plane of the substrate but also the inward forces exerted by the cells.7 As shown in Figure 2, Legant et al. developed a system to measure the forces exerted by cells within a three-dimensional (3D) matrix using TFM.7 Briefly, GFP-expressing cells were encapsulated in a PEG hydrogel (with known mechanical properties) containing fluorescent beads in the vicinity of each cell. Tracking the displacement of the beads within the surrounding matrix enabled linear elastic theory and finite element methods to be used to calculate the cellular traction forces.7 This powerful tool permits quantification of the cell–matrix interactions in a spatio-temporal manner, opening up many possibilities for the future study of various cell types, cell–ligand interaction studies, and even multicellular interactions.
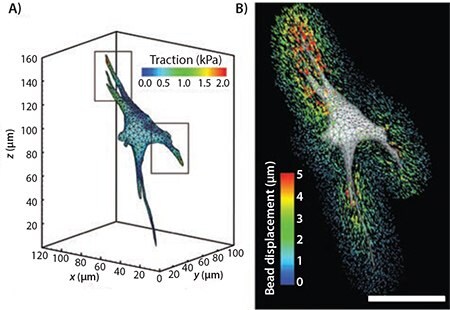
Figure 2. A) A contour plot of the magnitude of the traction forces exerted by a cell in a 3D hydrogel. B) A surface mesh of a cell shown with the bead displacement trajectories. Scale bar represents 50 μm. Adapted from Reference 6 with permission.
Cell Adhesion Force Microscopy (CAFM) is another method used to study cell–matrix interactions. Initially designed to measure the forces required to dislodge inanimate objects from surfaces, it was adapted to study cell– substrate adhesion forces. Developed by Sagvolden et al., this system uses an inclined Atomic Force Microscopy (AFM) cantilever and laser deflection to measure the force required to displace a cell adhered to a substrate.8 Combined with an inverted microscope, CAFM can generate force curves associated with dislodging adhered cells.8 This system eliminates the necessity of a gel-bead construct while easily integrating with a typical two-dimensional (2D) cell culture setup. However, CAFM is unable to provide the superior spatio-temporal resolution and 3D measurements of TFM.
Not surprisingly, many of the technologies for studying cellular mechanics have been adapted from other fields of study. For example, optical tweezers, originally developed in the field of physics to detect forces on micron-sized particles, have been adapted to study cellular mechanics. Specifically, the effects of the mechanical properties of the extracellular matrix on cell surface proteins can be studied using optical tweezers and microbeads. Choquet et al. first applied optical tweezers to manipulate ligand-coated latex microbeads to study their interaction with murine fibroblasts.9 This setup allowed tracking of individual microbead movement in response to cell force and the precise measurement of the forces required to detach beads from adhered cells.9 Wang et al. also used microbeads with magnetic twisting cytometry to study cell–ligand interaction. Ligand-coated ferromagnetic microbeads were used to study the effects of varying mechanical loads on specific integrins.10 In this system, instead of optical tweezers, a strong magnetic field was applied to the ferromagnetic microbeads. A second weaker magnetic field was added orthogonally to twist the microbeads.10 In this way, mechanical loading on a population of cells can be achieved at a higher throughput as compared to optical tweezers. As such, this system has the potential to study multicellular interactions.
In addition to microbead manipulation, micropads also allow insight into the interaction between a cell and its environment. Galbraith et al. built a force-measuring device consisting of laminin-coated micron-scale pads attached to cantilevers (Figure 3). Displacement of these pads in response to fibroblast-generated force was measured. Together with the known mechanical properties of the cantilevers, this setup allowed precise quantification of cell-generated force.11
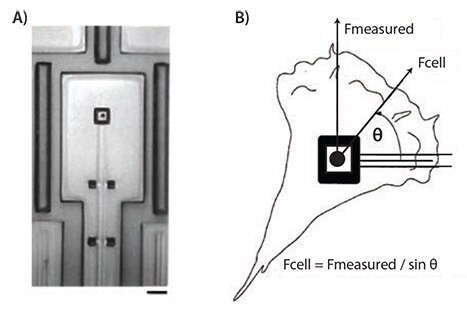
Figure 3.A) A micropad attached to a cantilever in a micromachined device used for studying cellular traction forces. Scale bar represents 10 μm. B) A force diagram illustrating the calculation of traction forces generated by a cell. Adapted from Reference 4 with permission.
The use of microstructures for understanding cellular interactions is not restricted to the study of physical forces. As seen in Figure 4, Pinney et al. demonstrated that microrods and microcubes affected the fibrotic response of cardiac cells.12 By modifying the microenvironment with which the cells interact, a therapeutic response could be achieved without the administration of drugs.12 Similarly, a nanotubular coating was shown to enhance endothelial healing while decreasing smooth muscle cell proliferation.13 These studies into the cellular interactions with micro- and nanostructures give insights to the effects of the physical environment on cellular functions.
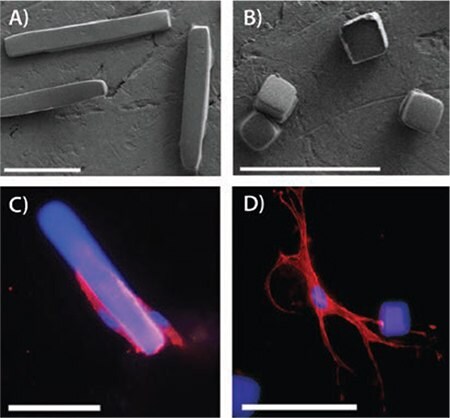
Figure 4.A) Microrods and B) microcubes fabricated from polymerized poly(ethylene glycol) dimethacrylate. Fluorescently stained murine cardiac fibroblasts interacting with microrods (C) and microcubes (D). Scale bar = 50 μm. Adapted from Reference 11 with permission.
The ingenious utilization of advanced materials and technologies has propelled our understanding of cellular interactions. As we continue to develop methodologies at the nanoscale and beyond, the opportunities for answering complex biological questions will continue to grow.
At the Microtissue Level: Characterization
Precise characterization of cellular interactions within a microtissue is necessary to support the advancement of tissue engineering. While biosensors have made substantial advancements in the characterization of biology at the cellular level, their sensitivity and resolution have been creatively applied to study the complexities of multicellular constructs. For example, fluorescent probes also lend themselves well to specific characterization of cell phenotype or biochemical signaling events in multicellular constructs because they require minimal disruption of the tissue or 3D construct. One example is the study of cell death at the center of multicellular spheroids—an important in vitro model used to study multicellular microtissues. Wartenberg et al. used confocal laser scanning microscopy to determine the cell viability in multicellular glioma spheroids.14 Even though diffusion, light scattering, and absorption of fluorescent dyes present significant limitations and data may be vulnerable to over-interpretation, fluorescent probes remain the best approach to characterizing multicellular construct geometry and viability without perturbing the cells or the structure of the microenvironment.
Inorganic micro- and nanoparticles have also demonstrated a versatility and utility in tissue-level sensing and imaging. Silica nanoparticles with a lipid bilayer coating have been used to great effect in improving fluorescent dye functionality for a variety of biosensing applications.15 Typically, liposomes rupture in the presence of silica microspheres, fusing around the microsphere and creating a lipid bilayer coating. Using porous silica microspheres, fluorophores can be incorporated into the internal pore space (Figure 5). Studies have shown that microspheres with a supported lipid bilayer can protect a pH-sensitive dye, maintaining fluorescence intensity in the presence of a higher pH in the surrounding medium.15 On the other hand, gold nanoparticles exhibit a higher intensity of absorption and scattering than most organic molecular dyes.16 These advantages, along with their size-dependent optical and photothermal properties, make gold nanoparticles suitable contrast agents for in vivo imaging. Gold nanoparticles, along with gold nanoshells, nanocages, and nanorods, can absorb near-infrared (NIR) light, which can penetrate human tissue to depths of a few centimeters. After absorbing NIR light, gold nanoparticles dissipate heat to the surrounding tissue, thus inspiring applications in imaging, cancer treatment, and tumor ablation.16
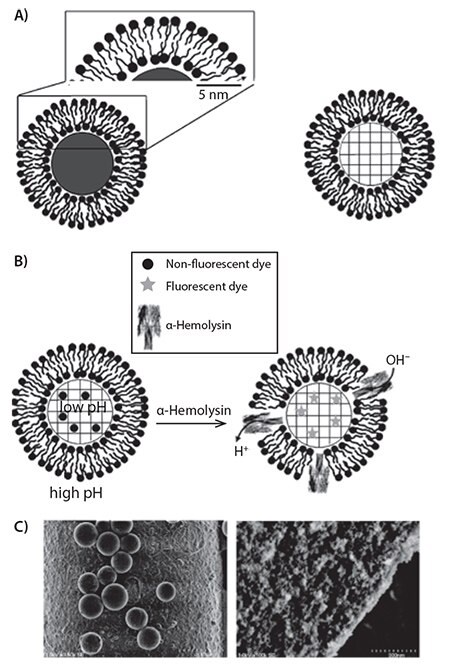
Figure 5.A) Schematic of a solid (left) or porous (right) silica microsphere with a lipid bilayer coating. B) One example of the use of porous silica microspheres. By incorporating alpha hemolysin, a pore is formed within the lipid bilayer, allowing a change in the internal pH and activation of the pH-sensitive dye. C) Scanning electron images of a nonporous microsphere (left) and a porous microsphere (right). High resolution of the porous microsphere details the porous surface. Adapted from Reference 14 with permission.
It is worth highlighting the application of inorganic particles in tissue engineering. Saldanha et al. used iron oxide particles to label and track mesenchymal stem cells (MSCs) using magnetic resonance imaging (MRI) for monitoring articular cartilage regeneration.17 Labeling MSCs with super-paramagnetic iron oxides prior to transplantation can facilitate longitudinal non-invasive in vivo bio-distribution assessment of transplanted cell populations (Figure 6B). The results of this study highlight the need to develop techniques to monitor cell-based tissue engineering strategies for a wide variety of applications.
At the Microtissue Level: Biosensors
Beyond the characterization of microtissues, micro- and nanotechnologies are actively used in the advancement of biosensors. Organic particulates and molecules can be specifically designed to act as biosensors, either as a recognition element for detection of enzymatic or antibody activity, or as a delivery scaffold for other recognition elements. Peptides or proteins conjugated to fluorophores, QDs, or nanoparticles have been used to detect HIV antibodies, bacterial components, and molecules in disease states, such as EGFR in cancer and amyloid plaques in Alzheimer’s patients.18 Polymer-based particulates are commonly developed into spheres and capsules for biosensor applications. One common polymer, poly(lactic-co-glycolic acid) (PLGA), is formed into nanoparticles via a double emulsion method, allowing for the incorporation of hydrophilic or hydrophobic cargo. Nehilla et al. created QD and drug-loaded PLGA nanoparticles for enhanced imaging and drug release applications (Figure 6A).19
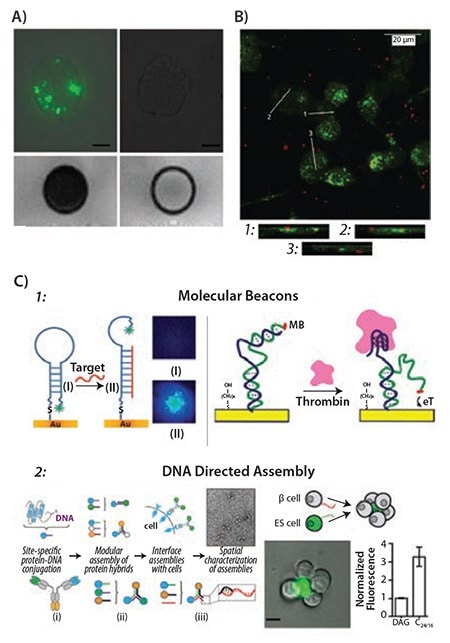
Figure 6. A) Uptake of fluorescently labeled micron-sized iron oxide particles in an MSC line. Left panel: Internalization of labeled particles into the MSC cytoplasm (top left) and successful MRI (2D gradient echo) contrast imaging (bottom left) of the same cells. Right panel: Unlabeled MSCs show no fluorescence (top right) or contrast imaging (bottom right). Scale bar = 10 μm. Adapted from Reference 16 with permission. B) QD-loaded PLGA nanoparticles added to a neuronal cell line (PC12) tissue culture. PC12 cells are labeled with CellTracker Green, while nanoparticles are shown in red. Adapted from Reference 18 with permission. C) Schematic examples of ssDNA biosensor applications. C1: Molecular beacons engineered to detect DNA (left), or the enzyme thrombin (right). C2: DNA-directed assembly of novel protein subunits (left) or novel multicellular aggregates (right). Adapted from References 20,21,23 with permission.
Aside from their applications as a nanoparticulate-based carrier, polymers themselves can be designed to act as biosensors. Common stimulusresponsive polymers include pNIPAM,20 pHEMA,21 and pVA-pAa.22 These polymers can be incorporated into hydrogels, polymer brushes, and molecularly imprinted polymer coatings to detect changes in analytes at either the cellular (DNA, FRET, antibody fragments) or multicellular (pH, glucose, and oxygen) levels.23 Oligonucleotides can also serve as platforms for biosensors. Typically, single-stranded DNA (ssDNA) is immobilized onto a surface such as glass, polymer, or gold nanoparticle via physisorption, chemical crosslinking, or covalent attachment. The phosphate groups of DNA can be used to detect positively charged analytes or dye molecules (Figure 6C).24 Furthermore, DNA strands can form secondary structures that act as molecular beacons to detect DNA, small molecules, and proteins for antibody detection and signal amplification (Figure 6C, left panel).24 Finally, ssDNA and its complimentary counterparts can be utilized toward DNA-directed assembly of novel antibodies25 and multicellular tissues26 which, in turn, can be used for further investigations in both biosensing and tissue characterization27 (Figure 6C, right panel).
Conclusion
It is clear for biologists and engineers that micro- and nanotechnologies are useful tools for studying cells and tissues in vitro. However, as it is becoming widely appreciated that the physico-chemical properties of the biomaterial used to study biological systems may affect the structure and functional performance of the system itself, it is important to understand the limitations and caveats of using such materials. For instance, at the multicellular level, the platform used to accommodate and monitor a tissue in vitro may trigger phenomena such as tissue inversion and compromise biological performance.28 The mechanisms behind how and why these phenomena happen are yet to be fully understood, but one thing is certain: if we continue the interdependent advancement of materials science and biology, there will be plenty of enlightening answers and engaging questions to follow.
Materials
References
如要继续阅读,请登录或创建帐户。
暂无帐户?