A Comparison of Superficially Porous Particle-based Column Chemistries for Peptide Mapping
Introduction
Superficially porous particles (SPP) have proven themselves as an efficient alternative to fully porous particles in HPLC separations. Higher efficiency per backpressure unit is achieved with these particles, in comparison with fully porous particles, and the advantages of this particle technology have been reported in the literature.1 These higher efficiencies are due to shorter diffusion paths within, and narrower particle size distributions of the SPP.
In this application, we compare three different superficially porous particle chemistries, from the BIOshell™ line of U/HPLC columns, in terms of their performance in the separation of peptides and peptide mapping. The quality parameters evaluated include peak width at half maximum (FWHM, full width half maximum), peak capacity, resolution between selected peak pairs, and theoretical plates (N).
For system suitability testing, a mixture of synthetic peptides in the MSRT Calibration Mix (MSRT1)was first used to compare the cyano, phenyl-hexyl, and C18 bonded phases, prior to performing the same comparisons with a tryptic digest of the monoclonal antibody reference material NISTmAb, a humanized IgG1k monoclonal antibody.
Experimental Methods
The system suitability mix, MSRT1, was prepared according to the instructions on the data sheet but with a final acetonitrile concentration of 1.6%. The injection volume was 10 µL.
Digestion of NISTmAb reference material 8671 was performed with a low artifact digestion buffer (EMS0011) using instructions provided in the product information sheet. To look for oxidized and deamidated forms of peptides, a digestion was also performed using overnight digestion in ammonium carbonate buffer. In this way, higher amounts of oxidized methionine and deamidated asparagine were generated on some peptides to evaluate chromatographic separations.
Instrument and gradient conditions are found in Tables 2-4.
Results and Discussion
A system suitability test mix is a recommended way to monitor the performance of a chromatographic system prior to submitting valuable samples for analysis. MSRT1 is a mix of 14 isotopically labelled peptides whose sequences are shown in Table 5. Each peptide is labelled with an isotopically labelled form of either [13C6, 15N1] leucine, [13C6, 15N2] lysine, or [13C6, 15N4] arginine (shown in brackets).
Table 5.MSRT1 peptide sequences. Labelled amino acids in brackets.
Injection of this mix on each of the three columns (Figure 1) indicates increasing retention of peptides in the order of cyano, phenyl-hexyl, and C18 under identical chromatographic conditions. The reason for this result most likely is derived from the retention mechanisms in the chromatographic system. Under the mobile phase conditions utilized, the analytes would behave mostly by a partitioning mechanism with retention increasing with the degree of hydrophobicity of the ligands on the silica surface. For the system suitability mix, the peptide sample solution consisted of 1.6% acetonitrile after following the instructions in the package insert. Even with this low organic solvent composition, some polar peptides were not retained on the cyano column even with the low 0.5% starting organic composition of the mobile phase. This result serves as a reminder to keep organic content as low as possible when introducing samples on the column, both for the mobile phase and the sample solution.
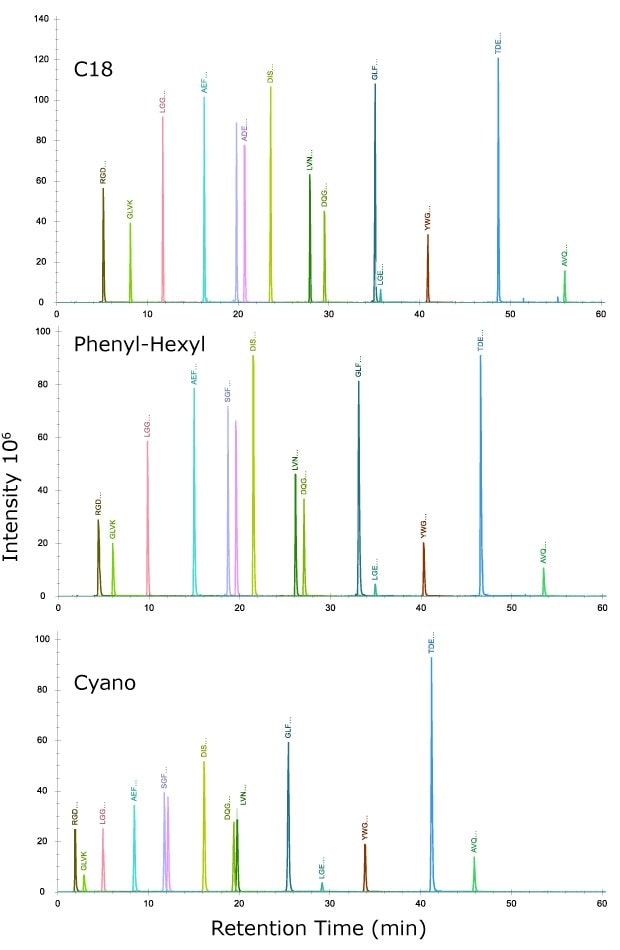
Figure 1.Injection of system suitability mix, MSRT1, containing 14 isotopically labelled peptides across molecular weights of 423.3 to 2176.1. The sequences of the peptides are provided in Table 5.
Peak widths are sharpest (full-width half max, FWHM) on the C18 column, ranging from 0.07 to 0.11 min, intermediate on the phenyl-hexyl at 0.09 to 0.18 min, and widest on the cyano at 0.12 to 0.16 min. Taking the average FWHM values for retained peptides yielded values of 0.093 for C18, 0.115 for the phenyl-hexyl, and 0.141 for the cyano (Table 6).
Using a peak capacity calculation of PC = 1+ tg/Wh, where tg is the length of the linear gradient, and Wh is the average FWHM, PC values were 643 for the C18, 524 for phenyl-hexyl and 426 for the cyano column. The average number of plates was also calculated using the equation N = 5.545(tr/FWHM)2 for each peak (tr = retention time of each peak) and then taking the average to yield the number of plates on the C18 column of 593,000, the phenyl-hexyl, 389,000, and the cyano, 159,000. These values reflect both the greater retention and sharper peaks obtained, overall, on the C18 column.
As another measure of column performance, several pairs of adjacent peaks were selected to calculate Resolution (R), where R = 2 Δ Z/ (WA+WB) and Z is the difference in retention time between the two peaks, WA, WB are the widths at the baseline of the two peaks.
The MSRT1 peak pairs selected were:
| Pair 1 | Peptides |
| 1 | RGDSPASSP[K] |
| 2 | GLV[K] |
| Pair 2 | |
| 5 | SGFSSVSVS[R] |
| 6 | ADEGISF[R] |
| Pair 3 | |
| 10 | GLFIIDD[K] |
| 11 | LGEYGFQNA[L] |
Figure 2 shows the separation achieved with these three pairs on the three column chemistries while the average resolution of the three pairs is shown in Table 6.
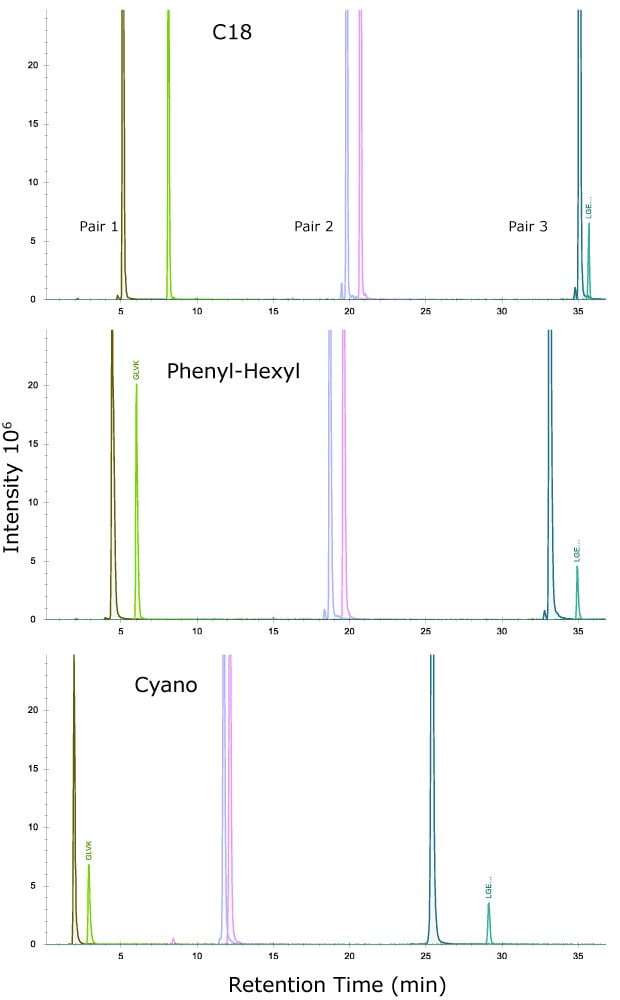
Figure 2.Separation of three peptide pairs from MSRT1 on the three column chemistries.
As seen, and perhaps most interesting, is the three columns have slightly different selectivities, particularly for the last eluting pair where the cyano column outperforms the C18 column and phenyl-hexyl column. This result illustrates the importance of phase chemistry when trying to achieve alternative selectivity of peptides. Due to the pi electrons in the triple bond of the cyano ligand, aromatic amino acids in the peptide may interact more through pi-pi stacking interactions than just through London dispersion forces as observed on the C18 column. The cyano column does not perform as well with Pairs 1 and 2, with Pair 2 not being fully resolved. The C18 and phenyl-hexyl columns both show good retention of Pair 1, the early eluting peptides.
The use of a system suitability mix can be recommended for regular evaluation of columns over time, to check system performance before submitting precious samples, and to make comparisons when evaluating new column chemistries.
Column Comparison with A Digested Monoclonal Antibody - NISTmAb
We next compared the columns using a tryptic digest of NISTmAb to create the separations for heavy chain (HC) and light chain (LC) peptides shown in Figure 3. Again, the same trend is observed in overall retention with C18 being the most retentive and cyano the least. All three columns performed equally well in terms of sequence coverage with values of 87% or greater for all three chemistries on the heavy chain and 97% coverage on the light chain. Peak widths at FWHM across all peptides in the digest gave equivalent results as the MSRT1 system suitability mix with C18 performing the best, followed by the phenyl-hexyl and then cyano columns.
The box and whisker plot in Figure 4 illustrates the distribution of peak widths obtained across the three columns for all HC and LC peptides. Interestingly, the broadest peak on each of the columns was a peptide containing three proline molecules. It has been reported2 that peptides containing prolines can suffer from broad peaks and peak splitting due to cis-trans isomerization of proline-proline bonds as well as other proline-amino acid bonds.
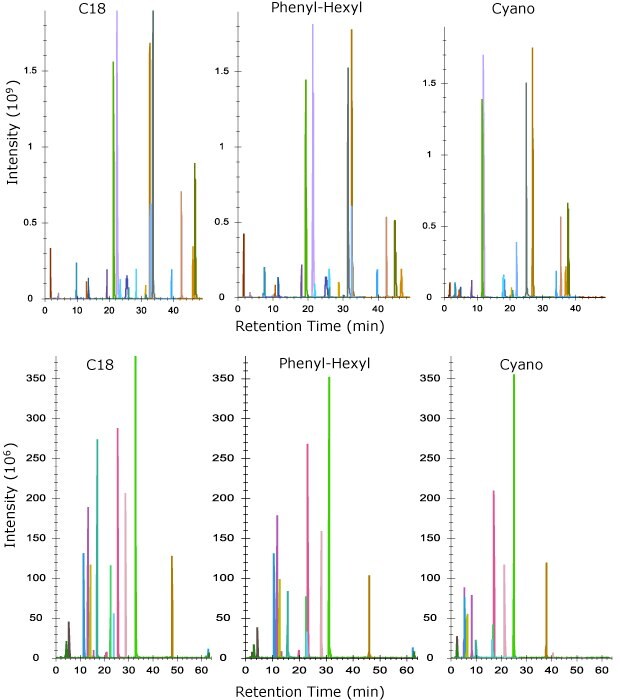
Figure 3.Elution profile of peptides from heavy chain (top) and light chain (bottom) using tryptic digest of NISTmAb on three different column chemistries shown.
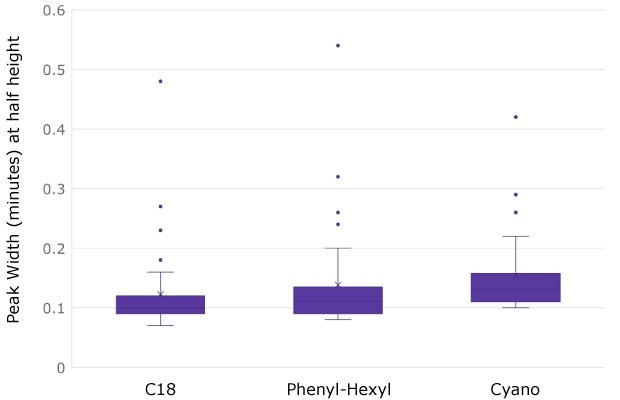
Figure 4.Peaks widths for NISTmAb peptides separated on three different column chemistries. The broadest peak observed on all columns is EPQVYTLPPSR due to three proline molecules and cis-trans isomerization (see text).
The number of theoretical plates was calculated based on the retention time and FWHM for all the heavy and light chain peptides as done previously with MSRT1 (Figure 5). The results again show the C18 column performing best followed by the phenyl-hexyl and then cyano chemistries.
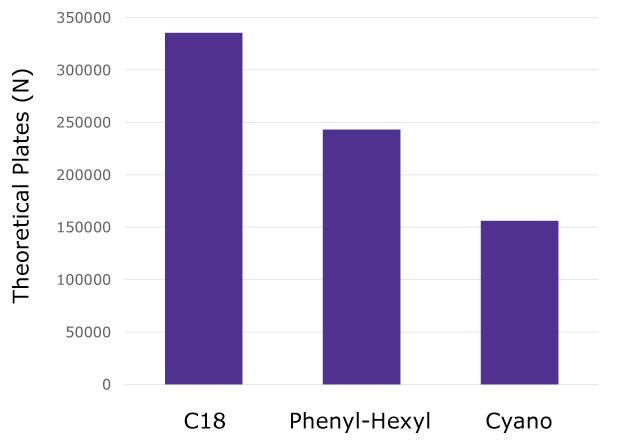
Figure 5.Average number of plates calculated from all NISTmAb heavy chain and light chain peptides on each column chemistry.
Selectivity comparison
A digestion of NISTMab was performed under conditions expected to yield greater amounts of deamidated asparagine as described. All three columns separated deamidated from unmodified peptide very well (Figure 6), allowing for the determination of this modification in quality control of therapeutic proteins. Separation of oxidized methionine on peptides resulted in those peptides eluting earlier than the native form by 2 minutes or more (not shown).
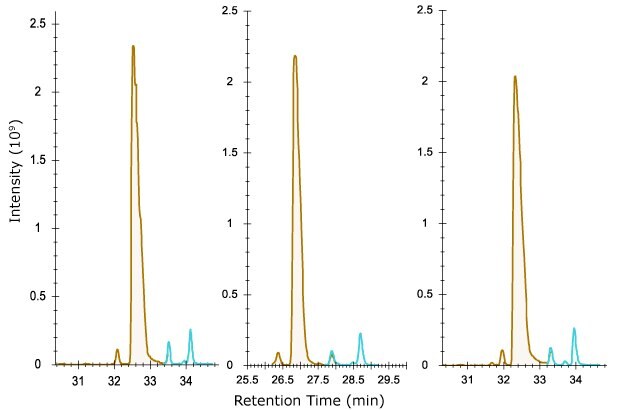
Figure 6.Separation of deamidated (blue) from unmodified NISTMab peptide FNWYVDGVEVHNAK (brown). The three deamidated forms are presumed to be a result of aspartic and isoaspartic acid isomers formed. Use of electron activated dissociation may aid in elucidating these identities.
Conclusions
Three superficially porous particles with different bonded chemistry were evaluated for their ability to perform peptide mapping-type experiments. Each column was of the same dimensions and operated under the same set of mobile phase and gradient conditions. A system suitability mix of 14 isotopically-labelled peptides was first used to evaluate peak widths, peak capacity, theoretical plates, and resolution of three peptide pairs. As expected, the C18 chemistry provided the best retention of peptides with the phenyl-hexyl phase next followed by cyano. Peak widths generally followed the same sequence with C18 again performing the best and, therefore, providing the highest peak capacity, and plates. Regarding the resolution of three selected peak pairs, it is apparent that the different phases do offer slight differences in selectivity so that, in some cases, a cyano or phenyl-hexyl chemistry may outperform a C18 phase. The use of MSRT1 has then proven to be useful in the performance evaluation of these columns.
The same trends were generally observed during the analysis of a monoclonal antibody digest, yet all performed equally well in terms of the sequence coverage provided. In this comparison, we use a relatively high concentration of a relatively pure mAb so that differences in sequence coverage achieved, from column to column, are not revealed as they might be with a more complex digest containing a range of protein concentrations. In a more complex sample, one might expect the C18 column to show better performance as a result of the better resolution, peak capacity, and plate number. The ability to separate deamidated forms of asparagine-containing peptides from the unmodified form is shown nicely in all three columns. Overall, the C18 chemistry provides the narrowest peaks, greatest retention, and highest peak capacity of the three columns. In some cases, evaluation of cyano and phenyl-hexyl chemistries may be desirable for the separation of critical peak pairs due to differences in selectivity.
Related Products
References
如要继续阅读,请登录或创建帐户。
暂无帐户?