Protein Structural Analysis
The function of a protein is directly dependent on its structure, its interactions with other proteins, and its location within cells, tissues, and organs. The structure and function of proteins is studied on a large scale in proteomics, which enables the identification of protein biomarkers associated with specific disease states and provides potential targets for therapeutic treatment. The understanding of protein structure and mapping of protein location, expression levels, and interactions yield valuable information that can used to infer protein function.
• Protein Structure
• Protein Structure Determination
• Protein Mapping
Featured Categories
Prestige Antibodies® developed using Human Protein Atlas data validated via various methods offer reliable research tools.
Learn about high-purity biological buffers in various formulations and packaging formats to get superior solution stability and pH control for your bioprocess workflow applications.
ISOTEC® Stable Isotopes are useful for tracer studies in proteomics and metabolomics, agents for MRI/MRS, and in a wide range of other biomedical applications.
We offer a wide range of cryo supplies including freezer racks, tubes & vials, labels & markers, and forceps for research and clinical applications from the most reliable names in the industry.
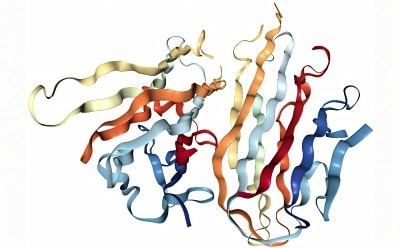
Protein structure
Protein structure is determined by the sequence of amino acids that compose the protein and how the protein folds into more complex shapes.
- Primary structure is defined by the amino acid sequence of the protein.
- Secondary structure is defined by local interactions of stretches of the polypeptide chain, which can form α-helices and β-sheets through hydrogen bonding interactions.
- Tertiary structure defines the overall three-dimensional structure of the protein.
- Quaternary structure defines how multiple protein subunits interact to form larger complexes.
Protein Structure Determination
The determination of three-dimensional protein structures at atomic resolution is useful in the elucidation of protein function, structure-based drug design, and molecular docking.
- NMR: Nuclear magnetic resonance (NMR) spectroscopy is used to obtain information about the structure and dynamics of proteins. In NMR, the spatial location of atoms is determined by their chemical shifts. For protein NMR, proteins are typically labeled with stable isotopes (15N, 13C, 2H) to enhance sensitivity and facilitate structural deconvolution. Isotopic labels are typically introduced by supplying isotopically labeled nutrients in the growth medium during protein expression.
- X-ray crystallography: Protein X-ray crystallography can be used to obtain the three-dimensional structure of proteins through X-ray diffraction of crystallized proteins. Crystals are grown by seeding highly concentrated protein in solutions that promote precipitation, with ordered protein crystals forming under suitable conditions. X-rays are aimed at the protein crystal, which scatters the X-rays onto an electronic detector or film. The crystals are rotated to capture diffraction in three dimensions, enabling calculation of the position of each atom in the crystallized molecule by Fourier Transform.
Protein Mapping
Mapping of the location and expression level of proteins in specific cells, tissues, and organs aids in the functional study of the proteome. Spatial distribution of proteins is key to protein function, with improper localization or expression triggering various disease states. Mapping projects such as the Human Protein Atlas provide a proteomic resource for biomarker discovery and aid in the understanding of disease pathology. Mapping of the interactome helps define the molecular interactions that occur on a cellular level, assisting in the understanding of protein function and providing valuable potential drug targets for disease.
Visit our document search for data sheets, certificates and technical documentation.
Related Articles
- Amino acid reference chart and products cater to diverse eukaryotic needs.
- The human protein atlas has an aim of mapping all human proteins within cells, tissues and organs and providing open-access information to advance understanding of human biology and disease.
- Information on Isoelectric Focusing including what it is and how it is used. In order to ensure the high performance of analysis, isoelectric point (pI) standards are needed.
- We presents an informational article concerning biomolecular NMR and the use of Isotope Labeling Methods for Protein Dynamics Studies.
- GPI anchored proteins, found in various organisms, are linked to membranes via phosphodiester linkages, crucial for cellular functions.
- See All (9)
Related Protocols
- This page covers the principles and methods of chromatofocusing, a chromatography technique that separates proteins according to differences in their isoelectric point (pI).
- Protein Structural Analysis
- Formaldehyde cross-linking protocol detects specific protein interactions with confidence.
Find More Articles and Protocols
How Can We Help
In case of any questions, please submit a customer support request
or talk to our customer service team:
Email custserv@sial.com
or call +1 (800) 244-1173
Additional Support
- Chromatogram Search
Use the Chromatogram Search to identify unknown compounds in your sample.
- Calculators & Apps
Web Toolbox - science research tools and resources for analytical chemistry, life science, chemical synthesis and materials science.
- Customer Support Request
Customer support including help with orders, products, accounts, and website technical issues.
- FAQ
Explore our Frequently Asked Questions for answers to commonly asked questions about our products and services.
To continue reading please sign in or create an account.
Don't Have An Account?