Regional Manufacturing for Supply Chain Security
Reimagining a Secure, Consistent, and Reliable Biopharmaceutical Supply Chain
Biopharmaceutical supply chains are vulnerable to a variety of destabilizing forces, from pandemics to natural disasters to a constantly changing geopolitical landscape. To ensure biomanufacturers can continue to operate regardless of global events, supply chains must be flexible and resilient, capable of managing instability before shortages occur. As a trusted supplier of the critical components you need for drug manufacturing, we reimagined supply chains to help you maintain business continuity. With regionalized manufacturing redundancy, our supply systems will weather instability and help future-proof your drug manufacturing.
Stay ahead by subscribing to our newsletter, which includes updates on our supply network, new products and services, and best practices.
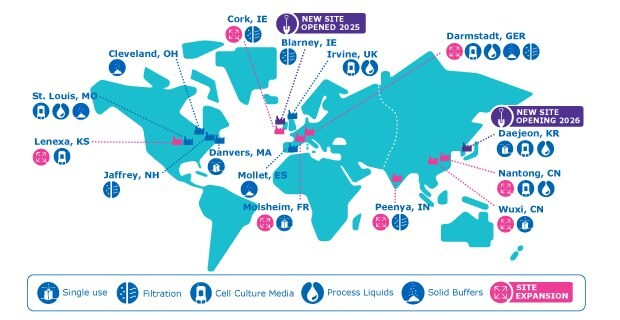
A Robust Regional Manufacturing Network with Global Oversight - Example of Filters, Cell Culture Media, Process Liquids and Buffers, and Single-use Technologies Manufacturing Networks for Drug Development and Manufacturing. This map features our five filter, six cell culture media, seven process liquids and buffers, and four single-use manufacturing sites providing you with access to consistent, high-quality products in each region (Americas, EMEA, and APAC).
Your patients rely on you, and you rely on us. That's why we reimagined our supply chain, with investments in regional manufacturing redundancy, bringing our products closer to you, creating resilient supply systems. With global oversight, you can rest assured you will always have access to consistent, high-quality products. Our supply chain management model is built upon the three pillars of security, consistency, and reliability, to ensure your business continuity.
Globally redundant regional manufacturing sites with personalized site qualification support facilitate access to the products you need.
Global oversight of new material sourcing and product development together with a global quality standard maintain product consistency across regional sites.
Global business continuity management and digital-enabled risk management for a robust, resilient, and reliable biopharma supply chain
Expanding Our Regional Impact
With over a dozen manufacturing facilities across the Americas, EMEA, and APAC, we fulfill the needs of biomanufacturers for filters, cell culture media, process liquids and buffers, and single-use technologies. Beyond our recently completed expansions in Lenexa (USA), Peenya (India), and Nantong (China), we are expanding our global network of regional manufacturing sites with additions to our Cork (Ireland), Darmstadt (Germany), Molsheim (France), and Wuxi (China) facilities. We recently opened a new filtration site in Blarney, Ireland and are building a new facility in Daejeon, South Korea. This global expansion is part of MilliporeSigma’s multi-year, €2 billion Life Science investment to meet the growing demand for biopharmaceuticals across Europe, China, and the United States.
Operating under our global business continuity management program, these regional manufacturing facilities offer several key benefits:
- Increased production capacity for our growing bioprocessing portfolio, with dedicated regionalized capabilities
- Equivalent product quality and performance standards across manufacturing sites
- Improved resiliency to ensure critical product availability in the event of a supply disruption
- Streamlined logistics and shorter transportation distances
Discover Our New Sites
Cell culture media, process liquids, and NovaSeptum® sampling systems.
Our new 44,500 m2 production center in Daejeon, South Korea, set to open in 2026, marks a significant expansion of our manufacturing capacities and capabilities for essential bioprocessing products.
Pellicon® 3 cassettes, Millipore Express® filters, Viresolve® Pro devices.
Our new 12,500 m2 regional manufacturing facility in Blarney, Ireland will manufacture filters for EMEA customers.
Explore Our Supply Networks
All our regional manufacturing centers operate under global standards ensuring consistent product performance irrespective of geographical location. This regional manufacturing redundancy provides local access to the high-quality products you trust, de-risking your supply chain.
Our cell culture media regional manufacturing centers in the US, the UK, Germany, China, and South Korea provide our customers across the globe with high quality customized and ready-to-use culture media.
Our filtration regional manufacturing centers in the US, Ireland, Germany, and India provide a best-in-class range of sterilizing-grade, virus and TFF filters.
Explore how we are transforming our single-use network, adding more capacities in Europe and Asia-Pacific, ensuring consistent and reliable production of high-quality single-use technologies in each region.
Our regional sites in the USA, Europe, and a new site in Asia-Pacific ensure our customers around the world always have access to high-quality process liquids, when and where they need them.
Similar to our current manufacturing network, filters, single-use components, cell culture media, process liquids and buffers manufactured at these new regional sites will be supported by Emprove® dossiers. These dossiers offer comprehensive documentation to facilitate your qualification, risk assessment, and process optimization, streamlining compliance.
Supply Chain Digitization
We believe in the transformative power of digitizing data to increase biopharma supply chain transparency and resilience and developed the eMERGE™ Standard eData Exchange Platform to automate delivery of analytical, quality, and logistical eData directly into your system, platform, or software. With the eMERGE™ platform, you can monitor, trend, and analyze critical production processes, applications, and use cases, enhancing operating efficiency, reducing downtime, while improving yield and on-time delivery of drug products.
Smart Manufacturing
We are modernizing our manufacturing network by implementing smart manufacturing technologies to enhance efficiency, adapt to changing demands, and improve flexibility.
For instance, the Daejeon site will continuously implement smart manufacturing technologies over time, leveraging digital technologies like Internet of Things (IoT), AI, and big data to optimize production processes. In addition to digital innovation, Daejeon will apply advanced technologies such as automated guided vehicles, automated warehouses, and industrial robots for operational efficiency. Key features will include interconnected machines and sensors for real-time data exchange to business operations systems, automation for increased efficiency, and data analytics for real-time data driven decision-making.
Sustainability Across the Value Chain
We are committed to a more sustainable future and take a holistic, life-cycle approach in our product design, packaging and manufacturing. While Blarney, Ireland is our first climate-neutral manufacturing site, facility upgrades at other sites have incorporated geothermal and photovoltaic systems, adiabatic cooling and air energy recovery exchangers to improve sustainability.
To continue reading please sign in or create an account.
Don't Have An Account?
