HPLC Analysis of Aripiprazole and Dehydro Aripiprazole Using an Ascentis® Express C18 Column
Materials
related product
Product No.
Description
Pricing
Dehydro aripiprazole solution
1.0 mg/mL in methanol with 5% 1 M HCl, ampule of 1 mL, certified reference material, Cerilliant®Aripiprazole solution
1.0 mg/mL (50:50 Methanol/Water with 1% 1N HCl), ampule of 1 mL, certified reference material, Cerilliant®used together
Product No.
Description
Pricing
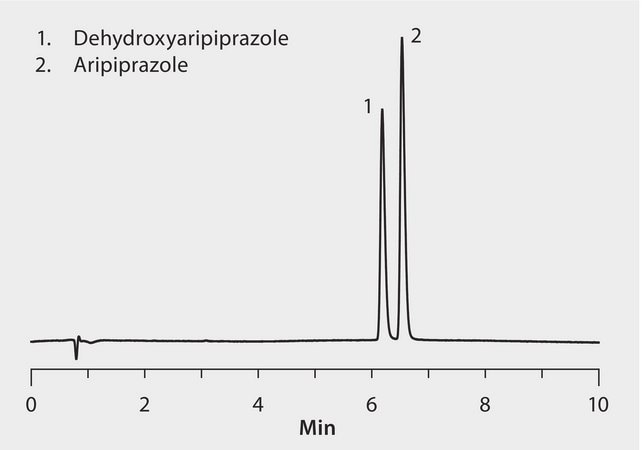
CONDITIONS
column
Ascentis Express C18, 10 cm x 3.0 I.D mm., 2.0 μm particles (50819-U)
mobile phase
[A] 10 mM ammonium formate; pH 3.0 with formic acid [B] 0.1% formic acid in methanol
gradient
0 to 40% B in 0.5 min; to 95% B in 3.5 minutes; held at 95% B for 2 min
flow rate
0.5 mL/min
pressure
6500 psi (445 bar)
column temp.
35 °C
detector
UV, 245 nm
injection
2 μL
sample
50 μg/mL in 10% methanol
Description
Analysis Note
Aripiprazole is marketed as the atypical antipsychotic Abilify® for the treatment of schizophrenia, bipolar disorder and clinical depression. Dehydroapiprazole is the primary metabolite of Aripiprazole.
Legal Information
Abilify is a registered trademark of Otsuka Pharmaceutical Co., Ascentis is a registered trademark of Merck KGaA, Darmstadt, Germany