HPLC Analysis of Z-Drugs on Ascentis® Express C18 2.0 μm
Materials
related product
Zaleplon solution
1.0 mg/mL in methanol, ampule of 1 mL, certified reference material, Cerilliant®Zolpidem phenyl-4-carboxylic acid solution
500 μg/mL in acetonitrile: water (1:1), ampule of 1 mL, certified reference material, Cerilliant®CONDITIONS
column
Ascentis Express C18, 10 cm x 3.0 mm I.D., 2.0 μm particles (50819-U)
mobile phase
[A] 10 mM ammonium formate pH 3.0 with formic acid in 90:10, water:acetonitrile; [B] 10 mM ammonium formate pH 3.0 with formic acid in 10:90, water:acetonitrile;
gradient
0 to 100% B in 3.0 min; held at 100% B for 0.5 min
flow rate
0.6 mL/min
pressure
5405 psi (373 bar)
column temp.
30 °C
detector
MS, ESI+, combined SIR
injection
0.5 μL
sample
10 μg/mL in 96:4, water:methanol
Description
Analysis Note
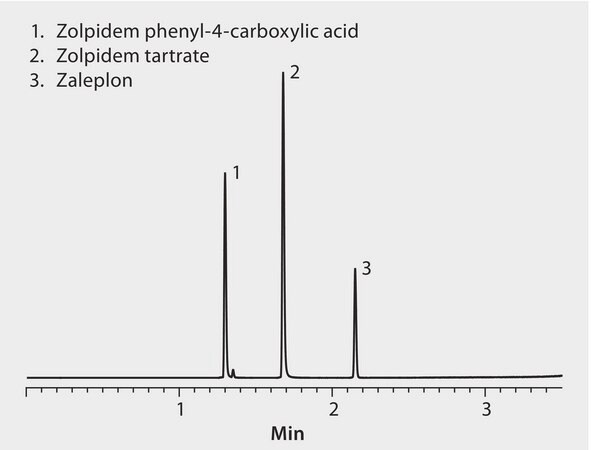
An rapid method for the simultaneous determination of the Z-drugs or sleep aids: zopiclone, zolpidem, and zaleplon is presented here. The need for greater analytical capacity and throughput for the analysis of sleep aid medicines (Z-drugs) in forensic toxicology laboratories can be met by the use of fast Ascentis Express 2.0 micron Fused Core UHPLC Columns.
Legal Information
Ascentis is a registered trademark of Merck KGaA, Darmstadt, Germany