LC/MS/MS Analysis of Immunosuppressant Drugs in Human Plasma on Ascentis® Express F5 after SPE Using HybridSPE®-Phospholipid
Materials
related product
Ascomycin solution
1.0 mg/mL in acetonitrile, ampule of 1 mL, certified reference material, Cerilliant®Cyclosporin A solution
1.0 mg/mL in acetonitrile, ampule of 1 mL, certified reference material, Cerilliant®Cyclosporin D solution
1.0 mg/mL in acetonitrile, ampule of 1 mL, certified reference material, Cerilliant®Sirolimus solution
1.0 mg/mL in acetonitrile, ampule of 1 mL, certified reference material, Cerilliant®Tacrolimus solution
1.0 mg/mL in acetonitrile, ampule of 1 mL, certified reference material, Cerilliant®used together
CONDITIONS
sample preparation
SPE (Solid Phase Extraction)
SPE tube/cartridge
HybridSPE-PLus 96-Well Plate, 50 mg/2 mL per well (575659-U)
sample addition
300 μL of 1% formic acid acetonitile, 100 μL spiked plasma per well
elution
apply vacuum at 10 in Hg for 4 min
column
Ascentis Express F5, 10 cm x 2.1 mm, I.D., 2.7 μm particles (50863-U)
mobile phase
[A] water; [B] acetonitrile:water (90:10), each with 10 mM ammonium formate
gradient
50% B for 0.5 min; to 80% B in 2 min; hold for 1.5 min; to 50% in 0.1 min; hold for 3 min
flow rate
0.3 mL/min
pressure
1300 psi (90 bar)
column temp.
75 °C
detector
MS, ESI(+), MRM mode
injection
2 μL
Description
Analysis Note
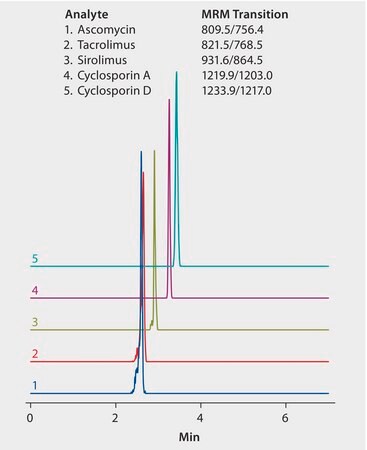
This work developed a reproducible LC/MS/MS and sample preparation method for determination of immunosuppressant drugs ascomycin, cyclosporin A, cyclosporin D, sirolimus and tacrolimus, in human plasma. HybridSPE®-PLus plates provided phospholipid-free plasma samples. UHPLC separation used an Ascentis Express F5 column. LC/MS grade solvents were used to supply low background interference and low particulate contaminants for robust, trouble-free operation. Cerilliant CRMs provided reliable identification and quantification.
Legal Information
Ascentis is a registered trademark of Merck KGaA, Darmstadt, Germany, HybridSPE is a registered trademark of Merck KGaA, Darmstadt, Germany