LC/MS/MS Analysis of Immunosuppressant Drugs on apHera™ C18
Materials
related product
Ascomycin solution
1.0 mg/mL in acetonitrile, ampule of 1 mL, certified reference material, Cerilliant®Cyclosporin A solution
1.0 mg/mL in acetonitrile, ampule of 1 mL, certified reference material, Cerilliant®Cyclosporin D solution
1.0 mg/mL in acetonitrile, ampule of 1 mL, certified reference material, Cerilliant®Sirolimus solution
1.0 mg/mL in acetonitrile, ampule of 1 mL, certified reference material, Cerilliant®Tacrolimus solution
1.0 mg/mL in acetonitrile, ampule of 1 mL, certified reference material, Cerilliant®CONDITIONS
mobile phase
[A] 0.1% formic acid water; [B] 0.1% formic acid in 98:2 (v/v) acetonitrile:water
gradient
50% B for 2 min; to 100% B in 2 min; held for 2 min
flow rate
0.4 mL/min
column temp.
65 °C
detector
MS, ESI(+), MRM
sample
300 ng/mL in (4:1) 1% formic acid acetonitrile:water
injection
5 μL
column
apHera C18, 15 cm x 2.1 mm I.D., 5 μm particles (56100AST)
Description
Analysis Note
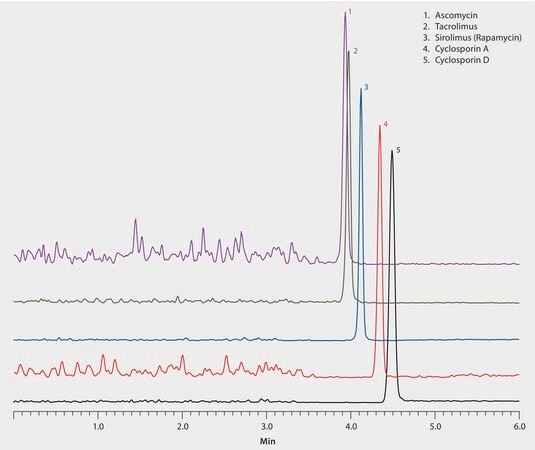
The apHera C18 HPLC column packed with polymeric particles demonstrated excellent peak shape and rapid elution for these bulky, immunosuppressant compounds under conditions not permitted by silica-based HPLC columns. The highest grade LC-MS solvents were used to supply low background interference and low particulate contaminants for robust, trouble-free operation. Cerilliant CRMs provided reliable identification and quantification.
Legal Information
apHera is a trademark of Sigma-Aldrich Co. LLC