LC/MS/MS of Digoxin and Digitoxin in Serum or Plasma on Titan C18 after SPE with HybridSPE®-Phospholipid, Minimization of Matrix Effects
Materials
related product
used together
CONDITIONS
sample preparation
SPE (Solid Phase Extraction)
sample/matrix
rat plasma or human serum spiked at 0.05 ng/mL with digitoxin and digoxin
SPE well plate
HybridSPE-PLus 96-well Plate, 50 mg/well (575659-U)
sample addition
100 μL spiked rat plasma followed by 300 μL 1% formic acid in acetonitrile
elution
apply vacuum
column
Titan C18, 10 cm x 2.1 mm I.D., 1.9 μm particles (577124-U)
mobile phase
10 mM ammonium formate in methanol:water (80:20)
flow rate
0.2 mL/min
pressure
4550 psi (314 bar)
detector
ESI-MS/MS
detector
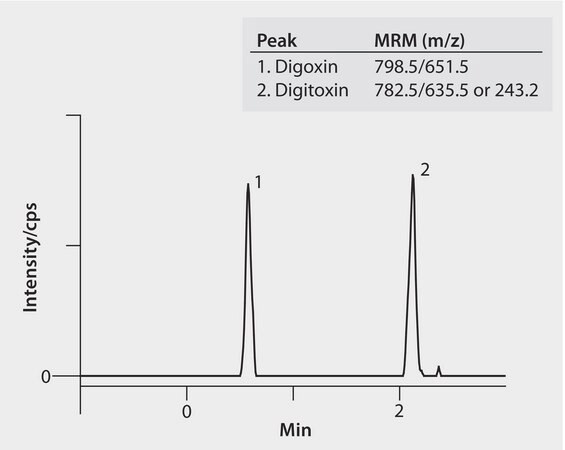
MRM Transitions: Peak MRM(m/z) (1.) Digoxin 798.5/651.5 (2.) Digitoxin 782.5/635.5 or 243.2
injection
2 μL
sample
0.05 ng/mL
Description
Analysis Note
The cardiac glycosides digitoxin and digoxin are widely prescribed for treating congestive heart failure. There is a need for simultaneous and sensitive determination of digitoxin and digoxin in biological fluids using LC/MS. This chromatogram shows the compounds at very low level of 0.05 ng/mL on a Titan C18 UHPLC column. The highest grade solvents provided clean, robust operation. Cerilliant CRMs and USP Reference Standards provided reliable quantification.
Legal Information
HybridSPE is a registered trademark of Merck KGaA, Darmstadt, Germany
null