UHPLC/MS Analysis of Anticonvulsants on Ascentis® Express HILIC
Materials
related product
Gabapentin solution
1.0 mg/mL in methanol, ampule of 1 mL, certified reference material, Cerilliant®Pregabalin solution
1.0 mg/mL in methanol, ampule of 1 mL, certified reference material, Cerilliant®Vigabatrin solution
1.0 mg/mL in methanol, ampule of 1 mL, certified reference material, Cerilliant®CONDITIONS
column
Ascentis Express HILIC, 10 cm x 3.0 mm I.D., 2.7 μm particles (53970-U)
mobile phase
[A] 5 mM ammonium formate pH unadjusted in 95:5, acetonitrile:water; [B] 5 mM ammonium formate pH unadjusted in 75:25, acetonitrile:water; (25:75, A:B)
flow rate
0.4 mL/min
pressure
1240 psi (85.5 bar)
column temp.
35 °C
detector
MS-ESI-, full scan 100-500 m/z
detector
5 μL
sample
3 anticonvulsants 1 μg/mL in 99:1, acetonitrile:methanol
Description
Analysis Note
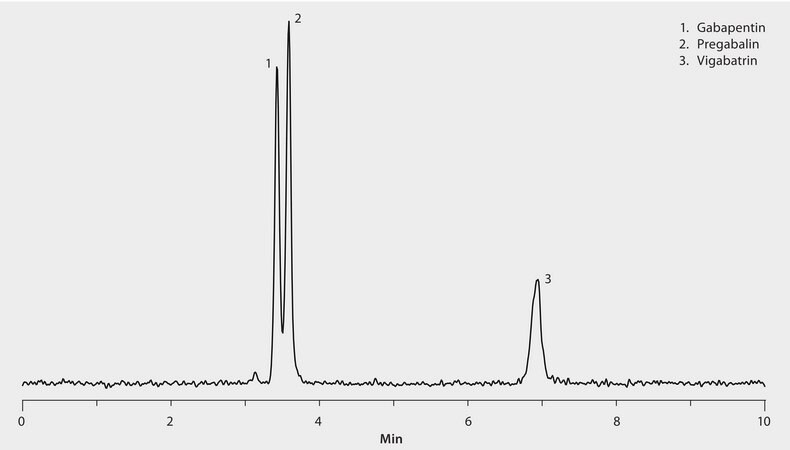
The unique selectivity of the Ascentis Express HILIC phase provided rapid LC/MS resolution of these anticonvulsant compounds. The highest grade LC-MS solvents were used to supply low background interference and low particulate contaminants for robust, trouble-free operation. Cerilliant CRMs provided reliable identification and quantification.
Legal Information
Ascentis is a registered trademark of Merck KGaA, Darmstadt, Germany