UHPLC Analysis of Aripiprazole and Its Primary Metabolite Dehydro Aripiprazole on Ascentis® Express C18
Materials
related product
Product No.
Description
Pricing
Dehydro aripiprazole solution
1.0 mg/mL in methanol with 5% 1 M HCl, ampule of 1 mL, certified reference material, Cerilliant®Aripiprazole solution
1.0 mg/mL (50:50 Methanol/Water with 1% 1N HCl), ampule of 1 mL, certified reference material, Cerilliant®used together
Product No.
Description
Pricing
CONDITIONS
column
Ascentis Express C18, 10 cm x 3.0 mm, 2.0 μm particles (50819-U)
mobile phase
[A] 10 mM ammonium formate in water, pH adjusted to 3.0 with formic acid [B] 0.1% formic acid in methanol
gradient
40% B for 0.5 min; to 95% B over 3.5 min; held at 95% B for 2 min
flow rate
0.5 mL/min
pressure
6500 psi (445 bar)
column temp.
35 °C
detector
UV, 245 nm
injection
2 μL
sample
50 μg/mL each compound in 10% methanol
Description
Analysis Note
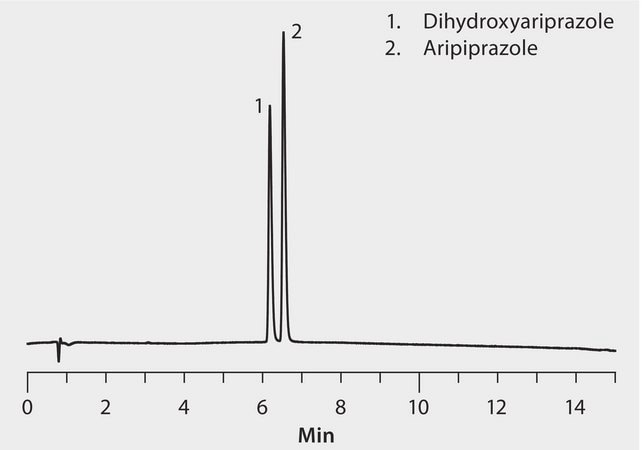
Aripiprazole is marketed as the atypical antipsychotic Abilify for the treatment of schizophrenia, bipolar disorder, and clinical depression. Shown here is the UHPLC separation of aripiprazole and a metabolite on a Fused-Core Ascentis Express C18 column. Cerilliant CRMs provided reliable quantification.
Legal Information
Ascentis is a registered trademark of Merck KGaA, Darmstadt, Germany