Extraction and Analysis of Lasix (Furosemide) in Horse Serum
A Case Study in SPE Method Development
The Office of Racing Commissioner’s (ORC) equine and human blood/urine testing programs play an important role towards ensuring the wagering public that drugs or other foreign substances are not administered to or present in any race horse, except as authorized by law. As a result, equine blood and urine samples are collected post-race and forwarded to such laboratories as Michigan Department of Agriculture (MDA) Equine Drug Testing Laboratory.
Lasix™ (furosemide) is a potent diuretic used to treat peripheral and pulmonary edema. For years it has been used to treat racehorses for exercise-induced pulmonary hemorrhage (EIPH) or bleeding. However, studies have suggested that there is a correlation between furosemide and improved racing performance prompting the horse racing industry to monitor the use of this drug.
In a cooperative effort with Dr. Brad Skiba, supervisor of the MDA Equine Drug Testing Laboratory, Supelco developed sample prep and analytical conditions for quantitating furosemide in horse serum via HPLC analysis. Using the systematic SPE method development approach illustrated in this report, a simple and highly selective SPE method was developed to recover 0.05-10.0µg/mL furosemide and indapamide (I.S.) from horse serum (Figure A). As a result of the systematic method development approach, a weaker elution solvent was determined allowing for direct analysis of the final SPE eluate (no evaporation and reconstitution). Recoveries were greater than 97% for all spike concentrations tested and RSDs averaged at 3.4%
Figure A. Structures of furosemide and indapamide
Systematic SPE Method Development
In SPE, selectivity is defined as the ability of the sorbent and the extraction method to discriminate between the analyte(s) of interest and endogenous interferences within the sample matrix. In reversed-phase SPE, selectivity is typically governed by two main variables: pH and percent organic modifier. When conducting SPE method development systematically, two or three experiments are typically performed with the use of standards. By using standards, one can track the location of the analytes throughout the SPE process relative to systematic changes in key variables influencing analyte retention and elution. Below is a list of key advantages of systematic SPE method development.
Advantages of Systematic SPE Method Development
- Excellent quantitative precision and accuracy
- Improved recovery and sensitivity
- Improved method ruggedness
- Application specific knowledge for more effective method transfer and troubleshooting
Sample Characteristics and Phase Selection
Furosemide is a moderately acidic compound with a ~pKa of 6. Note that furosemide is extensively bound to serum proteins (albumin). To disrupt drug-protein interactions, samples must be acidified prior to extraction.
Indapamide was chosen as the internal standard because of its structural similarity to furosemide.
Horse serum is an aqueous portion of blood from which plates, corpuscles, and clotting factors (fibrinogen) have been removed. Endogenous interferences that should be considered include proteins such as albumin and globulins, lipids, salts and carbohydrates. It is therefore important to determine which phase chemistries offer the best selectivity towards the compounds of interest, and the solvent strength limit of the wash eluent before premature elution occurs.
The aqueous nature of the sample matrix and hydrophobic character of the analytes offer an excellent opportunity for reversed-phase retention. Also, known retention from HPLC experiments and adequate logP values indicate that C18 SPE may be a good choice for this application.
In this study, SPE method development was conducted on Discovery DSC-18 SPE cartridges (50mg/1mL).
Load Optimization
1mL working standards containing 5.0µg/mL of each of both furosemide and indapamide (I.S.) in 10mM KH2PO4, pH 3 (adjusted with H3PO4) were loaded onto Discovery DSC-18 SPE tubes (50mg/1mL) previously conditioned and equilibrated with 1mL methanol and DI H2O. The load flow-through eluate was then collected and analyzed via HPLC-UV. A lack of analyte presence in the load eluate indicated that adequate retention was observed under aqueous and acidic conditions.
Wash/Elution Profile
To determine application specific wash/elute parameters, 7 Discovery DSC-18 SPE cartridges (50mg/1mL) were conditioned and equilibrated with 1mL methanol and DI H2O, and loaded with 1mL of the 5.0µg/mL furosemide/indapamide test mix. The respective tubes were washed/eluted with 1mL of test solvents ranging from 0-100% methanol. The wash/elute eluate was collected and analyzed via HPLC-UV. A graph relating organic strength to peak area response was used to identify breakthrough (Figure B).
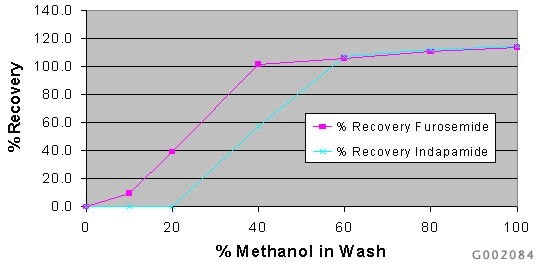
Figure B. Results of the wash/elute profile for furosemide and indapamide on discovery DSC-18 SPE.
By profiling the major parameters affecting analyte retention and elution, application specific guidelines have been established for defining, optimizing, and troubleshooting an extraction method. From the results of the wash/elute profile, we learned that furosemide elutes between 0-10% methanol, and indapamide commences breakthrough at 20-40% methanol. To fully recovery both compounds, 60% methanol is required.
Incorporation of Serum Sample Matrix
Horse serum was incorporated into a method defined by data generated from precursory load optimization and wash/elute profile studies.
Systematically Developed Method for Extracting Furosemide and Indapamide (I.S.) Using Discovery DSC-18 SPE
- Condition Discovery DSC-18 SPE tube (50mg/1mL) with 1mL MeOH
(n = 3 for ea. concentration) - Equilibrate DSC-18 SPE tube with 1mL 10mM KH2PO4, pH 3
(adjusted with H3PO4) - Load 1mL Sample (Table 1)
- Wash with 1mL 10mM KH2PO4, pH 3 (adjusted with H3PO4)
- Elute with 1mL 60% MeOH in DI water
- Directly analyze eluate (no evaporation + reconstitution) via HPLC. Calculate Recovery and RSDs for ea. sample.
Quantitation
Working calibration standards not subjected to sample preparation were analyzed via HPLC-UV. Response factors ([Peak Area Furosemide]/[Peak Area Indapamide (I.S.)]) were plotted against nominal concentration (0.05-10µg/mL) using a weighted (1/x) linear regression model (Figure C). Relative recovery and standard deviations for each of the furosemide concentrations extracted were determined through response factor curve interpolation (Table 2).
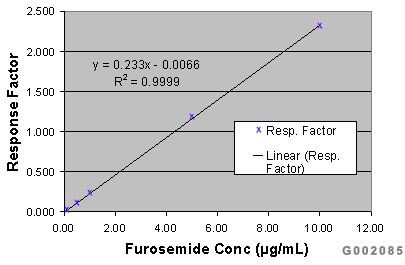
Figure C. Calibration curve for furosemide/indapamide peak area response factors.
Simpler SPE Procedure, Cleaner Extracts, & Reduced Analytical Run Times
Most reversed-phase SPE protocols using C18 require a 100% organic elution. Buffer is then added to the organic eluate, or the eluate must be evaporated and reconstituted with mobile phase prior to LC resolution. This adds an additional step and further prolongs the extraction procedure. Using the systematic SPE method development approach, we were able to determine a weaker solvent (60% methanol) to elute furosemide and indapamide (I.S.) without sacrificing recovery. This allowed for direct injection/analysis of the final eluent reducing the number of extraction steps and minimizing overall processing time.
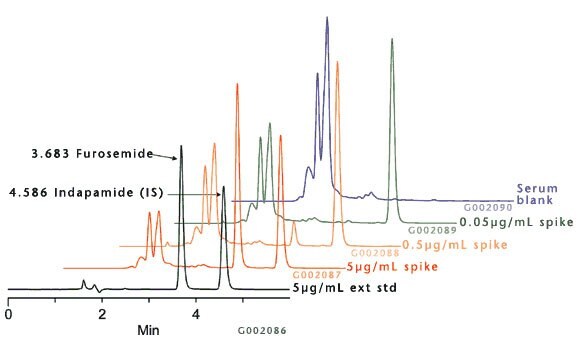
Because wash solvents stronger than water or buffer may cause premature analyte elution, a more selective/weaker eluate was necessary for maintaining adequate sample clean-up. This is illustrated in Figure D where the systematically developed DSC-18 method provided clean extracts signified by chromatograms with low background deficient of misleading peaks/responses. The clean extracts allowed for minimal analytical run times (6 min.) resulting in faster more accurate results.
Excellent Recovery and Reproducibility
On the Discovery DSC-18 SPE cartridge using the systematically developed method, average relative recovery and RSD (Table 2) for furosemide at the 5 concentrations was 100.2 ± 3.4%.
Note that the procedure is quantitative down to 0.5µg/mL serum. Below this level, reasonable precision is evident; however, accuracy suffers. This is mainly due to the detection limitations of UV absorbance for furosemide. Fluorescent detection or mass spectrometry is likely to provide increased sensitivity. Other strategies for increasing sensitivity include increasing the SPE sample volume and scaling to 3mL SPE tubes. If this is not viable, decreasing the SPE elution volume or increasing the HPLC injection volume (>10µL) are viable solutions for increasing sensitivity and accuracy.
Conclusion
In SPE, selectivity is defined as the sorbent/extraction method’s ability to discriminate between the analytes of interest and endogenous interferences within the original sample. By approaching SPE method development systematically on phases of varying selectivity, key variables influencing analyte retention and elution can easily be tested and tracked when using standards. As a result, one can strategically manipulate and make quick adjustments to the extraction method to meet the sample prep objectives during the life of the assay.
Materials
Trademarks
- Lasix is a trademark of Hoechst Aktiengesellschaft
- Discovery is a registered trademark of Sigma-Aldrich Co. LLC
To continue reading please sign in or create an account.
Don't Have An Account?