Single-walled Carbon Nanotubes: Exfoliation and Debundling Procedure
Single-walled Carbon Nanotubes Dispersion
The dispersibility and bundle defoliation of single-walled carbon nanotubes (SWCNTs) (724777, 773735, and 775533, for example), which can be applied to materials produced by the CoMoCAT® process, have been extensively investigated by SouthWest NanoTechnologies (SWeNT®) and at the University of Oklahoma.1 The procedure employed to disperse SWCNTs has a significant impact on the final suspension characteristics, including solution concentration, stability, and the fraction of individual nanotubes. Even though it is possible to obtain a stable dispersion of bundles where the majority of bundles are not exfoliated, the specific aim is to create dispersions of exfoliated tubes. A number of technical approaches, including covalent and non-covalent stabilization of SWCNTs, can be adopted to prepare a stable and homogeneous dispersion of SWCNTs. The non-covalent approach has been of particular interest since the surface structure and properties of the nanotubes remain intact when this methodology is used.
In a typical dispersion procedure, a surfactant is adsorbed on the SWCNT surface and the solution is sonicated, leading to the debundling or exfoliation of the carbon nanotubes. Since an individual nanotube covered by surfactant molecules has a density similar to water, centrifugation at 20,000–170,000 x g is needed to separate carbonaceous impurities and large SWCNT bundles from isolated SWCNTs. Smaller bundles may be separated with an optional second sonication step, which can further increase the fraction of individual tubes in the suspension (see Figure 1).
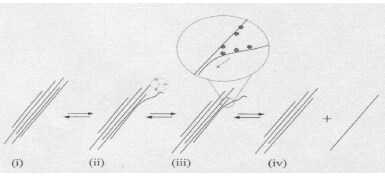
Figure 1.Proposed mechanism of nanotube isolation from bundles(2).
Preparation Instructions
Surfactant Stock Solution – Prepare a sodium cholate hydrate (C6445) solution in deionized water (2 mg/mL). Alternative surfactants include sodium deoxycholate (D6750) and sodium dodecyl sulfate (SDS, 436143).
- Ultrasonic processing “frays” the bundle end.
- The bundle end becomes a site for additional surfactant adsorption.
- Process continues in an “unzippering” fashion.
- Process terminates with the release of an isolated, surfactant-coated nanotube in solution.
Dispersion Procedure
- Add 1–2 mg of SWCNT to 7 mL of the Surfactant Stock Solution.
- Sonicate for 1 hour at a power density of 1 Watt/mL, with a horn sonicator (Z528897 or Z509302 for 110 V, or Z511471 or Z511463 for 220 V) and a microtip (Z192740). The solution should be cooled during sonication.
Note: Over-sonication can shorten the SWCNTs. - Centrifuge the mixture for 30 minutes at a relative centrifugal force (RCF) of 25,000–32,000 x g. In addition to dispersing SWCNTs, centrifugation can enhance product quality by removing graphite, residual catalyst, and amorphous carbon, as well as large bundles into the pellet. These impurities are minimal in the SWeNT materials.
Note: Longer centrifugation times and higher RCF values can remove smaller bundles and narrow the distribution in the final suspension. - Dilute the surfactant liquid with Surfactant Stock Solution until the absorption at 780 nm is less than 1 AU in a standard 1 cm path length optical glass cuvette using an instrument that has a double beam spectrometer and a tungsten filament bulb.
References
To continue reading please sign in or create an account.
Don't Have An Account?