Atomic Layer Deposition of Nanomaterials for Li-Ion Batteries, Fuel Cells, and Solar Cells
Introduction
Nanomaterials are considered a route to the innovations required for large-scale implementation of renewable energy technologies in society to make our life sustainable. This holds both for energy harvesting and for energy storage devices. In recent years, this has spurred many research activities on 0D (nanoparticles), 1D (nanowires and nanotubes), and 2D (such as graphene) materials—which can serve as elemental building blocks of device elements—while there also has been a lot of emphasis on 3D nanostructuring in general. Many challenges arise when building devices from nanomaterials, particularly in the preparation of these nanomaterials as well as in their modification, functionalization, and stabilization.
Vapor phase deposition processes are one class of methods that can be used to address these challenges. For example, nanoparticles, nanowires, nanotubes, and graphene can all be grown from the vapor phase. This also holds for thin films that can be used to modify, functionalize, and stabilize the nanomaterials or to build nanostructured materials. One vapor phase deposition technique that is receiving growing attention is atomic layer deposition (ALD). The application of this technique for building energy harvesting and energy storage devices will be addressed in this article. In the next sections, the preparation of thin films and nanoparticles by ALD will be addressed and recent progress in the application of ALD-prepared nanomaterials in Li-ion batteries, fuel cells, and solar cells will be briefly reviewed on the basis of selected examples.
Atomic Layer Deposition of Thin Films and Nanoparticles
ALD is a cyclic vapor phase deposition technique in which precursors and reactants are injected into the reactor chamber alternately (see Figure 1A) and in which the reactions are driven by the surface chemistry and not by thermal decomposition.1 A prerequisite for ALD is that the surface chemistry in the halfreactions of the ALD cycles is self-limiting. This allows for a precise growth control with Angstrom-level resolution as well as an excellent uniformity and conformality on demanding substrate topologies. In addition, ALD affords the deposition of high-quality materials at rather low substrate temperatures. Typical substrate temperatures range from 400 °C down to room temperature, depending on the material and the process. Moreover, ALD is a scalable technology that has already been implemented in the manufacturing of hard-disk read heads, memory, and logic devices.
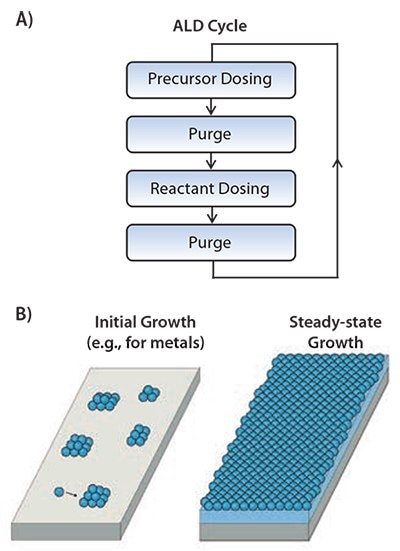
Figure 1. A)Schematic representation of an ALD cycle consisting of two half-cycles in which precursor and reactant dosing are alternated and separated by purge steps. B) Initial and steady-state growth conditions during ALD leading to nanoparticles (e.g., for metal ALD processes) and closed, conformal films, respectively.
ALD leads to layer-by-layer growth of amorphous or polycrystalline thin films (see Figure 1B) under regular circumstances. Such a layer is deposited every cycle and corresponds to less than a monolayer of material as typical “growth-per-cycle” values range from 0.25 to 1.5 A. However, during initial growth the process can deviate significantly from the steady-state growth conditions. Growth can be inhibited or in some cases even enhanced depending on the substrate, its pretreatment, and the material deposited.2 For example, for metals deposited on oxide or oxidized substrates, it has clearly been established that initial growth takes place in the Volmer-Weber mode instead of layer-by-layer mode. This means that, initially, islands of metal atoms are formed (see Figure 1B) which grow in size every ALD cycle due to sticking of atoms directly from the gas phase or indirectly by surface diffusion processes.3 After a certain number of cycles, the islands start to coalesce and a closed film is obtained. This implies that metal nanoparticles can also be deposited via ALD by exploiting the island growth in the initial ALD cycles. The ALD cycles need to be halted before coalescence occurs and the size of the nanoparticles can be accurately controlled by carefully selecting the number of cycles. Figure 2 illustrates the opportunities provided by ALD in terms of depositing nanoparticles and conformal films, both on planar substrates as well as on carbon nanotubes, nanowires, and 3D-structured substrates.
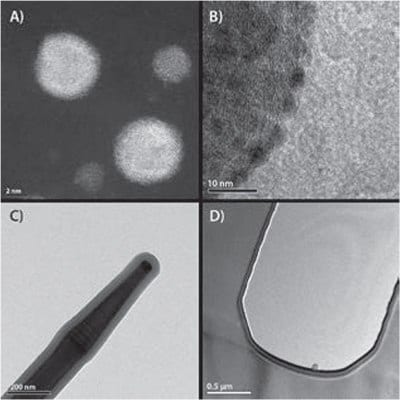
Figure 2.Examples of nanoparticles and films deposited by ALD on various substrate materials. A) Core/shell nanoparticles of Pd/Pt on a planar oxide substrate. B) Pd nanoparticles on a multiwall carbon nanotube. C) GaP nanowire covered by thin film of Al2O3. D) Trench in silicon covered by a stack of Al2O3/TiN/Al2O3/TiN. The scale bars in A)–D) are 2 nm, 10 nm, 200 nm, and 0.5 μm, respectively.
Li-Ion Batteries, Fuel Cells, and Solar Cells
Lithium-Ion Batteries
Li-ion batteries are currently considered as energy storage solution for a wide range of applications, from electrical vehicles to microsystems. To reach improvements in terms of capacity, power, and lifetime, there is a trend toward nanostructuring of the electrode materials while there are also several other efforts to vigorously change the battery configuration.4 The latter includes, for example, all-solid-state Li-ion batteries built from thin films, where 3D structuring is also proposed. ALD is considered an enabling technology for several concepts of nanostructured Li-ion batteries.5
In Figure 3, the potential of ALD is schematically illustrated for three battery concepts; i.e., particle-based electrodes, 3D-structured electrodes, and 3D all-solid-state microbatteries. Electrodes in commercial batteries are most often based on micrometersized particles of active materials, which are mixed with binder compounds and conductive additives. Figure 3A illustrates a particle-based cathode. The liquid electrolyte penetrates the porous network, facilitating the Li+ transport. When going to smaller particle sizes to increase the surface-to-volume ratio and hence the power capablity, the decomposition of the electrolyte by the so-called solid electrolyte interphase (SEI) formation can also be increased. This adverse effect can be reduced or possibly prevented by modifying the surface of the particles by applying a passivating or protective film. Such films need to be ultrathin to have high Li+ and electron conductivities while excellent conformality is needed to be sufficiently protective. Researchers from the University of Colorado at Boulder and the National Renewable Energy Laboratory (NREL) have successfully pioneered the protection of particles by ALD-prepared films. For example, improved stability was demonstrated by depositing Al2O3 ( 718475 and 634875) on LiCoO2 (442704) particles.6 It was also shown Al2O3 improves the cycle-life of natural graphite anodes.7 In the latter case, it was found the best cycle-life was not obtained by directly coating the individual particles but by first composing the electrode and carrying out the ALD cycles with Al(CH3)3 (663301) and H2O dosing afterward. Furthermore, the improvement in stability was obtained for extremely thin layers (i.e., only a few ALD cycles) which might indicate the positive effect may not require a closed Al2O3 film.7
ALD has also been used in 3D-structured electrodes, in which the electrode is specifically designed such that diffusion paths in the materials are short, which improved transport of electrons and Li+. Figure 3B shows an example of such a structure. Nanowires of Al serve as current collectors and allow for easy transport of electrons to the active material deposited on the nanowires. Moreover, Li+ transport is facilitated by the open structure in which the electrolyte can penetrate easily. This design was taken by Cheah et al. who coated the Al nanowires with TiO2 using ALD.8 The TiO2 was used as the anode electrode material. TiO2 has a relatively high redox potential, alleviating problems with electrolyte decomposition. Similarly, Kim et al. created a hollow TiO2 nanonetwork by depositing a thin film of TiO2 on a peptide assembly. This assembly was subsequently removed by a high temperature step which also resulted in crystallization of the TiO2 film into the anatase phase.9 In both cases, a high capacity and high rate capability were observed.
There is a growing interest in Li-ion microbatteries for which the challenge is to achieve a high energy density at small device dimensions.4 The designs for such microbatteries are typically allsolid- state as packaging methods do not scale very well. Moreover, for all-solid-state batteries, electrolyte decomposition issues are greatly reduced, which extends the cycle-life. The batteries consist of thin films with limited thickness due to the low Li+ and electron diffusion in solid materials. 3D structuring is considered necessary to increase the storage capacity without deteriorating the power capacity. Notten et al. proposed a system-in-package solution consisting of a 3D topology etched into Si substrates in which the battery stack is deposited10 (see Figure 3C). In this stack, the electrolyte separates the electrodes, which in turn are both connected to the electrical circuit by current collectors. Furthermore, the battery stack needs to be encapsulated from the environment and a Li-diffusion barrier is necessary to avoid loss of Li into the Si substrate. Since all layers need to be deposited as thin films in the 3D topology, ALD or very conformal CVD processes are required to build such a battery stack. The first stage of the research on this kind of microbatteries focused on the deposition of passive battery materials. It was, for example, demonstrated that TiN (595063) deposited by ALD from TiCl4 (697079) and H2-N2 plasma was well-suited as a Li-diffusion barrier and current collector even though the TiN was not fully conformal.11 Furthermore, current collectors of Pt were prepared successfully by ALD from MeCpPtMe3 (697540) and O2 gas, although it was found the Pt needed special surface preparation to achieve a good adhesion.5
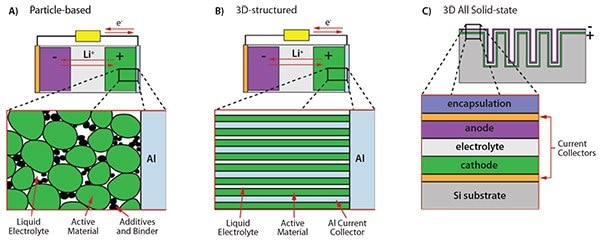
Figure 3. Various configurations of Li-ion batteries involving ALD-prepared films. Li-ion battery with A) particle-based electrode in which the particles are covered by an ultrathin ALD film for protection/stabilization against the liquid electrolyte and B) a nanostructured electrode to reduce Li and electron diffusion paths, to absorb volume changes to avoid cracking and crumbling of the material, and to increase storage capacity due to nanosize effects. In A) and B), the liquid electrolyte is able to penetrate into the porous electrode structures. C) An all-solid-state Li-ion battery consisting of a stack of active and passive thin film battery materials. A 3D-structured substrate is used to increase the storage capacity per unit of surface area and this requires conformal deposition techniques such as ALD.
In the second stage of the research, active battery materials were considered. For example, electrochemical testing revealed Co3O4 (637025, 203114, and 221643) deposited by ALD from CoCp2 (339164) and O2 plasma is a good thin film anode material.12 In follow-up work, it was demonstrated ALD-prepared LiCoO2 was electrochemically active as an anode material.13 This material was deposited by combining Co3O4 and Li2CO3 (752843) deposition (from LiO t-Bu and O2 plasma) in so-called supercycles, and electrochemical activity was achieved after crystallization of the material by hightemperature annealing. In recent years, several more Li-based materials have been deposited by ALD processes, although experiments in which the materials are electrochemically active are still relatively rare. This holds for both the Li-containing cathode and electrolyte materials as well as for the anode materials. Another challenge is the deposition of these materials in 3D topologies as well as the demonstration of functional microbattery devices.
Fuel Cells and Solar Cells
Similar nanostructuring approaches as the ones for Li-ion batteries are being considered for other energy technology devices such as fuel cells and solar cells. Research on solid oxide fuel cells (SOFCs), containing a solid metal oxide as electrolyte, concentrates on reducing the operating temperature from traditional values near 1,000 °C to lower temperatures of 500–800 °C. These high temperatures are required for conduction of oxygen ions through the metal oxide which acts as a membrane. One approach is the reduction of the resistance of the electrolyte by decreasing its thickness. To investigate electrolytes at nanometer scale thickness, several groups deposited yttria-stabilized zirconia (YSZ) (734683 and 774049) by ALD supercycles of Y2O3 (774022 and 544892) and ZrO2 (774030 and 544760).14 With respect to solar cells, many applications of ALD-prepared thin films have been considered in accordance with the variety in solar cell devices.15,16 Excellent passivation of the surfaces of crystalline silicon solar cells has been achieved by ALD Al2O3 films. This has even lead to industrial ALD reactors based on the spatial ALD method in order to reach the high throughput required.17 Copper indium gallium (di) selenide (CIGS) solar cells can benefit from the application of ALD buffer layers which are Cd-free. Zinc oxide compounds, such as (Zn,Mg)O and Zn(O,S), as well as films of In2S3 (308293) have mainly been considered for this purpose.18 In dye-sensitized solar cells ALD films have been applied as a compact layer to prevent the backward electron transfer from TCO to the electrolyte.19 Furthermore, much research has been carried out on barrier layers for various photoanode structures. For instance, TiO2 nanoparticles (700347, 700355, and 700339) coated uniformally with thin passivating Al2O3 films resulted in improved solar cell performances which are related to the high recombination energy barrier of the Al2O3-TiO2 interface, the high work function of the Al2O3 barrier, and the low energy barrier between the dye and Al2O3.19,20 Furthermore, ALD films are also excellent moisture permeation barriers that have been successfully used to encapsulate flexible CIGS and organic solar cells.21
All aforementioned examples of applications of ALD in fuel cells and solar cells concerned thin films. There are, however, some cases in which nanoparticles synthesized by ALD can be used in such devices. For example, fuel cells require catalyst layers for reduction and oxidation reactions at the electrode/electrolyte interface. ALD Pt can be used for these applications, not only as ultrathin films22 but also as nanoparticles. An example of a direct methanol fuel cell (DMFC) with nanoparticles as a catalyst is shown in Figure 4A. In such a DMFC, methanol is oxidized and O2 is reduced on catalyst particles leading to CO2 and H2O. These catalyst particles can be mono- or bimetallic nanoparticles of Pt and/or Ru. The nanoparticles are typically supported on carbon black but, alternatively, a carbon nanotube network structure can be used. Nanoparticles can be deposited on and into this carbon nanotube network structure by ALD.23 Similarly, in dye-sensitized solar cells Pt nanoparticles (773875) prepared by ALD can be used. Pt is typically applied on the counter electrode (e.g., a transparent conductive oxide) by sputtering or electrodeposition and it catalyzes the electrolyte reduction process from I3- to 3I through the electrons arriving from the external circuit. However, for flexible dye-sensitized solar cells a transparent counter electrode is required to enable back-side illumination because preparation of the nanocrystalline TiO2 photoanode is incompatible with the temperature-sensitive transparent polymer foils. This implies the Pt layer at the counter electrode needs to have both a high catalytic activity and a high transparency. This can only be reached by employing a low, yet efficient loading of well-dispersed nanoparticles. These can be deposited by ALD, albeit with an adapted process to enable low temperature processing.24
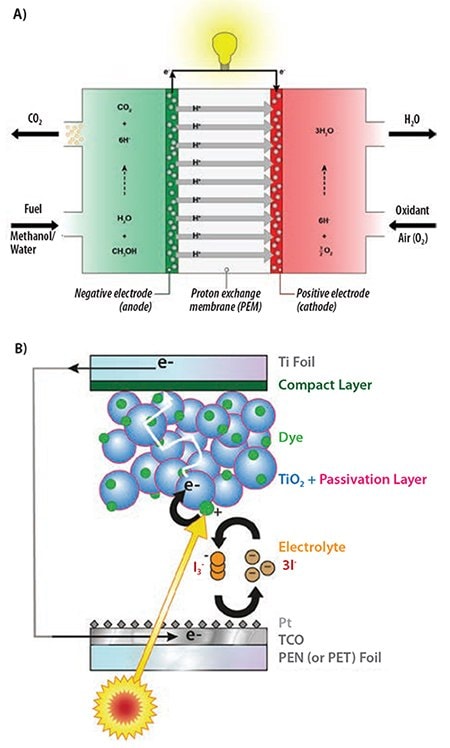
Figure 4.Applications that can benefit from the deposition of noble metal nanoparticles by ALD. A) Direct methanol fuel cell with electrodes containing noble metal nanoparticles. The nanoparticles can be deposited on and into the electrode material by ALD. B) A flexible dye-sensitized solar cell built up from polymer and metal foils. Light is entering from the “back side” of the cell through the counter electrode. A high transparency can be obtained by a counter electrode consisting of a transparent conductive oxide decorated with ALD Pt nanoparticles.
Summary
Selected examples of the recent progress in the application of ALD to Li-ion batteries, fuel cells, and solar cells have been addressed here. This progress clearly demonstrates ALD is an enabling technology to synthesize, modify, functionalize, or stabilize high-performance nanomaterials used in energy harvesting and storage devices. It also highlighted that ALD cannot only be used to deposit thin films, but it is also a promising technique for the synthesis of metal nanoparticles. Furthermore, it is noted ALD is a scalable method. Scalable methods are a prerequisite for manufacturing when aiming at the wide-scale implementation of energy technologies in our society. However, ALD has yet to provide a track record in low-cost, high-throughput processing. This means that other methods might be preferable to ALD when such alternative methods become available. In such cases, ALD can still be of key importance as it can serve as a method to demonstrate technological feasibility of devices and provide an excellent starting point for model studies.
Acknowledgment
Prof. Peter Notten of the Eindhoven University of Technology (TU/e), Dr. Leif Christensen of the Danish Technological Institute (DTI), and Dr. Thomas Brown of the University of Rome–Tor Vergata and their co-workers are acknowledged for the scientific discussions and collaborations. The members of the Plasma & Materials Processing group (TU/e) are thanked for their contributions to the research and a special thank you goes out to Dr. Marcel Verheijen (TU/e) for the electron microscopy images.
Materials
References
To continue reading please sign in or create an account.
Don't Have An Account?