Enzymatic Assay of α-Amylase (EC 3.2.1.1)
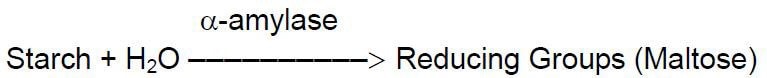
Unit Definition: One unit will liberate 1.0 mg of maltose from starch in 3 minutes at pH 6.9 at 20 °C.
Reagents and Equipment Required
- Sodium phosphate, monobasic, Product No. S0751
- Sodium chloride, Product No. S9888
- Starch from potato, Product No. S2004
- Sodium hydroxide, Product No. S5881
- Potassium sodium tartrate, tetrahydrate, Product No. S2377
- 3,5-Dinitrosalicylic acid, Product No. D0550
- D‑(+)‑Maltose, monohydrate, Product No. M9171
Precautions
Please consult the Safety Data Sheet for information regarding hazards and safe handling practices.
Preparation Instructions
Use ultrapure water (≥18 MΩ×cm resistivity at 25 °C) for the preparation of reagents.
Buffer solution (20 mM Sodium Phosphate with 6.7 mM Sodium Chloride, pH 6.9 at 20 °C) – Prepare a solution containing 2.4 mg/mL of sodium phosphate, monobasic, Product No. S0751, and 0.39 mg/mL of sodium chloride, Product No. S9888, in ultrapure water. Adjust to pH 6.9 at 20 °C using 1 M NaOH/1 M HCl.
Starch solution [1.0% (w/v) Soluble Starch Solution] – Prepare a 10 mg/mL solution using starch from potato, Product No. S2004, in Buffer:
- Solubilize the solution by boiling on a heating/stir plate for 15 minutes with mixing.
- Remove from heat. Continue to mix the solution and allow to cool to room temperature.
- Bring the solution to final volume with ultrapure water.
- Continue mixing the solution throughout the assay procedure.
2 M Sodium Hydroxide (NaOH) solution – Prepare a 80 mg/mL solution using sodium hydroxide, Product No. S5881, in ultrapure water.
5.3 M potassium sodium tartrate, tetrahydrate solution – Prepare a 1,496 mg/mL solution of potassium sodium tartrate, tetrahydrate, Product No. S2377, in 2 M Sodium Hydroxide (NaOH) solution. Dissolve solids by heating on a heating/stir plate with mixing. Do not heat to a boil!
96 mM 3,5-Dinitrosalicylic acid solution – Prepare a 21.9 mg/mL solution using 3,5‑Dinitrosalicylic acid, Product No. D0550, in ultrapure water. Dissolve solids by heating on a heating/stir plate with mixing. Do not heat to a boil!
Color Reagent solution – For preparation of 40 mL, add:
- 12.0 mL of warm (50–70 °C) ultrapure water to an appropriate size amber bottle.
With mixing, slowly add: - 8.0 mL of warm 5.3 M potassium sodium tartrate, tetrahydrate solution
- 20 mL of warm 96 mM 3,5-Dinitrosalicylic acid solution
This solution is stable for 6 months at ambient temperature if protected from light. Volume prepared may be adjusted if needed.
0.2% (w/v) Maltose Standard – Prepare a 2 mg/mL solution in a volumetric flask using D‑(+)‑maltose, monohydrate, Product No. M9171, in ultrapure water.
α-Amylase Sample solution – Immediately before use, prepare a solution containing 0.75‑1.5 units/mL of α-Amylase in 20 °C ultrapure water.
Procedure
Final assay concentration – In a 2.00 mL reaction volume, the final concentration is 0.50% (w/v) starch and ~1 unit of α-amylase.
1. α-Amylase Sample Assay
a. Pipette (in mL) the following reagents into suitable containers:
b. Mix by swirling and equilibrate to 20 °C. Then add (in mL):
c. Mix by swirling and incubate for exactly 3.0 minutes at 20 °C. Then add:
d. Cover containers with a vented cap and place in a boiling water bath for exactly 15 minutes.
e. Remove containers from boiling water bath. Then add (in mL):
f. Cool solutions on ice to room temperature.
g. Then add (in mL):
h. Mix by inversion. Blank a suitable spectrophotometer against air at 540 nm and record the A540 for the Samples and Sample Blank.
2. Standard Curve Preparation
a. Prepare a standard curve by pipetting (in mL) the following reagents into suitable containers:
Note: Additional standard levels may be added as needed.
b. Cover containers with a vented cap and place in a boiling water bath for exactly 15 minutes.
c. Remove containers from boiling water bath. Cool solutions on ice to room temperature.
d. Then add (in mL):
e. Mix by inversion. Blank a suitable spectrophotometer against air at 540 nm and record the A540 for the Standards and Standard Blank.
Results
Calculations
- Determine the ΔA540 of each Standard vs. the Standard Blank.
ΔA540 (Standard) = A540 (Standard) – A540 (Standard Blank) - Prepare a standard curve by plotting the ΔA540 of the standards vs. mg of maltose using linear regression.
- Determine the ΔA540 of each Sample vs. the Sample Blank.
ΔA540 (Sample) = A540 (Sample) – A540 (Sample Blank) - Determine the mg of Maltose released using the standard curve.
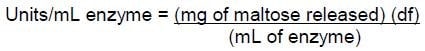
where:
df = dilution factor
mL enzyme = mL of Sample added in step 1b.
6.
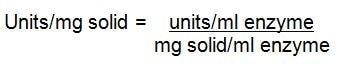
Related Products
Reference
To continue reading please sign in or create an account.
Don't Have An Account?