Buffer Exchange and Desalting for Affinity Chromatography
Dialysis is frequently mentioned in the literature as a technique to remove salt or other small molecules and to exchange the buffer composition of a sample. However, dialysis is generally a very slow technique, requiring large volumes of buffer. During handling or as a result of proteolytic breakdown or non-specifc binding to the dialysis membranes, there is a risk of losing material. A simpler and much faster technique is to use a desalting column, packed with Sephadex G-25, to perform a group separation between high and low molecular weight substances. Proteins are separated from salts and other small molecules.
In a fast, single step, the sample is desalted, transferred into a new buffer, and low molecular weight materials are removed.
Desalting columns are used not only to remove low molecular weight contaminants, such as salt, but also for buffer exchange before or after different chromatographic steps and for the rapid removal of reagents to terminate a reaction.
Sample volumes up to 30% of the total volume of the desalting column can be processed. Sample concentration does not influence the separation as long as the concentration of proteins does not exceed 70 mg/mL when using normal aqueous buffers. The sample should be fully dissolved. Centrifuge or flter to remove particulate material.
For small sample volumes, it may be possible to dilute the sample with the buffer that is to be used for chromatographic purifcation, but cell debris and particulate matter must still be removed.
To prevent possible ionic interactions the presence of a low salt concentration (25 mM NaCl) is recommended during desalting and in the fnal sample buffer.
Volatile buffers such as 100 mM ammonium acetate or 100 mM ammonium hydrogen carbonate can be used if it is necessary to avoid the presence of NaCl.
Figure 76 shows a typical buffer exchange and desalting separation. The process can be monitored by following changes in UV absorption and conductivity.
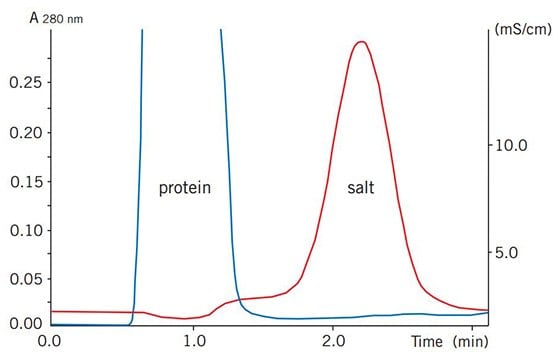
Figure 76.Buffer exchange of mouse plasma (10 mL) on HiPrep™ 26/10 Desalting.
For laboratory scale operations, Table 16 shows a selection guide for prepacked, ready to use desalting and buffer exchange columns.
To desalt larger sample volumes:
- Connect up to 5 HiTrap® Desalting 5 mL columns in series to increase the sample volume capacity, e.g. 2 columns: sample volume 3 mL, 5 columns: sample volume 7.5 mL.
- Connect up to 4 HiPrep™ 26/10 Desalting columns in series to increase the sample volume capacity, e.g. 2 columns: sample volume 30 mL, 4 columns: sample volume 60 mL. Even with 4 columns in series, the sample can be processed in 20 to 30 minutes, at room temperature, in aqueous buffers.
Instructions are supplied with each column. Desalting and buffer exchange can take less than 5 minutes per sample with greater than 95% recovery for most proteins.
Alternative 1: Manual desalting with HiTrap® Desalting 5 mL using a syringe
- Fill the syringe with buffer. Remove the stop plug. To avoid introducing air into the column, connect the column “drop to drop” to the syringe (via the adapter provided).
- Remove the twist-off end.
- Wash the column with 25 mL buffer at 5 mL/min to remove completely the 20% ethanol (supplied as storage buffer). If air is trapped in the column, wash with degassed buffer until the air disappears. Air bubbles introduced onto the column by accident during sample application do not influence the separation.
- Apply the sample using a 2–5 mL syringe at a flow rate between 1–10 mL/min. Discard the liquid eluted from the column.
- If the sample volume is less than 1.5 mL, change to buffer and proceed with the injection until a total of 1.5 mL has been eluted. Discard the eluted liquid.
- Elute the protein with the appropriate volume selected from Table 16.
Collect the desalted protein in the volume indicated.
Note: 5 mL/min corresponds to approximately 120 drops/min when using a HiTrap® 5 mL column. A simple peristaltic pump can also be used to apply sample and buffers.
The maximum recommended sample volume is 1.5 mL. See Table 17 for the effect of reducing the sample volume applied to the column.
A simple peristaltic pump can also be used to apply sample and buffers.
Alternative 2: Simple desalting with ÄKTAprime plus
ÄKTAprime plus contains pre-programmed templates for individual HiTrap® Desalting 5 mL and HiPrep™ 26/10 Desalting columns.

Buffer Preparation
Prepare at least 500 mL of the required buffer.
- Follow the instructions supplied on the ÄKTAprime plus cue card to connect the column and load the system with buffer.
- Select the Application Template.
- Start the method.
- Enter the sample volume and press OK.
Figure 77 shows a typical result obtained from ÄKTAprime plus. The UV (protein) and conductivity (salt) traces enable pooling of the desalted fractions.
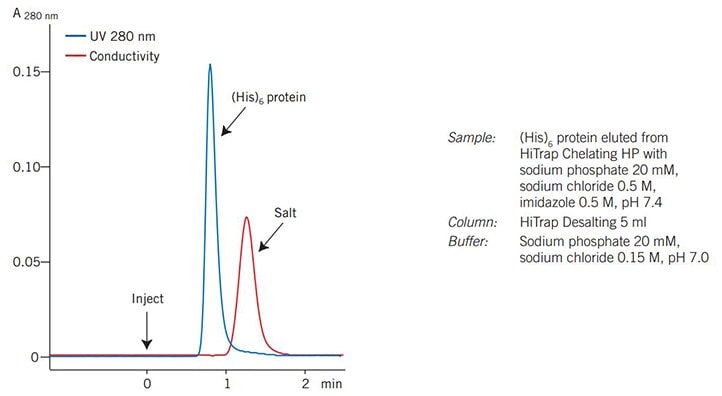
Figure 77.Desalting of a (His)6 fusion protein on ÄKTAprime plus.
Materials
To continue reading please sign in or create an account.
Don't Have An Account?