Selectivity and the Properties of a HIC Medium
Section Overview
While ligands contribute significantly to the degree of hydrophobicity of a medium, the matrix can also influence the final selectivity. Chromatography media for hydrophobic interaction are made from porous matrices, chosen for their physical stability, their chemical resistance to stringent cleaning conditions and their low level of non-specific interaction.
Matrix
An optimal balance between porosity and particle size offers a large surface area covered by ligands and so ensures a high binding capacity. High porosity with an open pore structure is an advantage when separating large biomolecules.
- An inert matrix minimizes non-specific interactions with sample components.
- High physical stability ensures that the volume of the packed medium remains constant despite extreme changes in salt concentration or pH, thus improving reproducibility and avoiding the need to repack columns.
- High physical stability and uniformity of particle size facilitate high flow rates, particularly during cleaning or re-equilibration steps, to improve throughput and productivity.
- High chemical stability ensures that the matrix can be cleaned using stringent cleaning solutions if required.
- Modern HIC media use either polymeric or agarose-based matrices to fulfill the requirements for chemical and physical stability, high binding capacity and different particle sizes (Table 1).
A SOURCE™ matrix is made from polystyrene with divinyl benzene to produce highly spherical (monodispersed), small (15 μm), porous particles (Figure 1) that facilitate high-resolution separations at high flow rates.
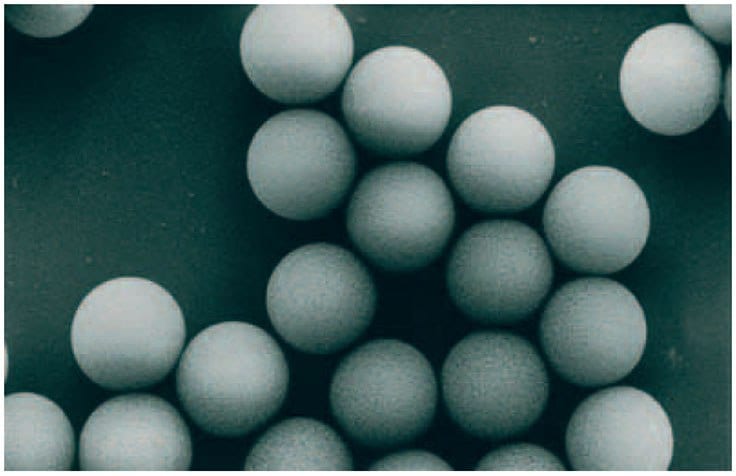
Figure 1.Electron micrograph of SOURCE™ showing spherical, monodispersed particles.
Sepharose® matrices are based on hydrophilic chains of agarose, arranged in bundles and with different degrees of intra-chain cross-linking (Figure 2), to give a range of rigid, macroporous matrices with good capacity and low non-specific binding. The most suitable matrix can be selected according to the degree of resolution, binding capacity and flow rates required. For example, gradient elution on Sepharose® High Performance (34 μm) will give a high-resolution separation whereas the larger particles of Sepharose® Fast Flow (90 μm) are best suited for high capacity, step elution at high flow rate.
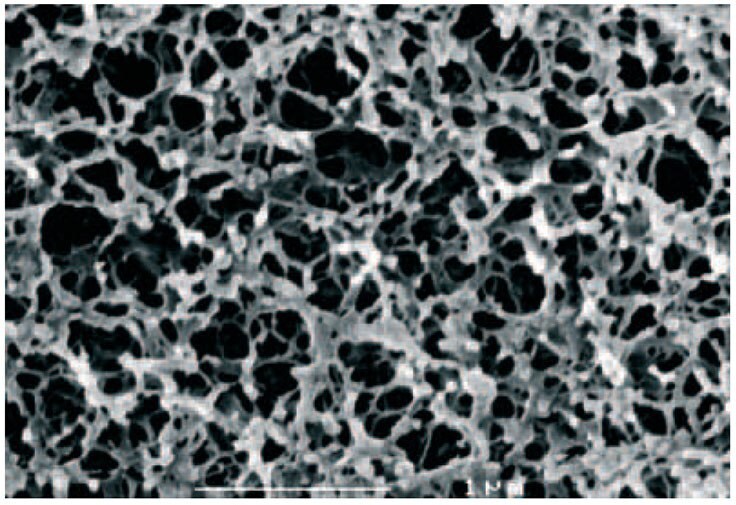
Figure 2.Structure of cross-linked agarose media (Sepharose®).
Different matrices for HIC media have been used over the years, and references to these will still be found in scientific literature, for example, Sepharose® CL-4B substituted with phenyl or octyl ligands. However, more recently developed matrices offer greater physical and chemical stability. To benefit from significantly faster separations and improved performance, it may be worth testing for selectivity on new media and re-optimizing old protocols.
Ligands and degree of substitution
The ligand and the degree of ligand substitution on a chromatography matrix also contribute to the final hydrophobicity of the medium and hence to the selectivity. Figure 3 shows an example of a protein mixture separated on the same Sepharose® Fast Flow matrix, but with four different ligand conditions: phenyl (high substitution), phenyl (low substitution), butyl and octyl.
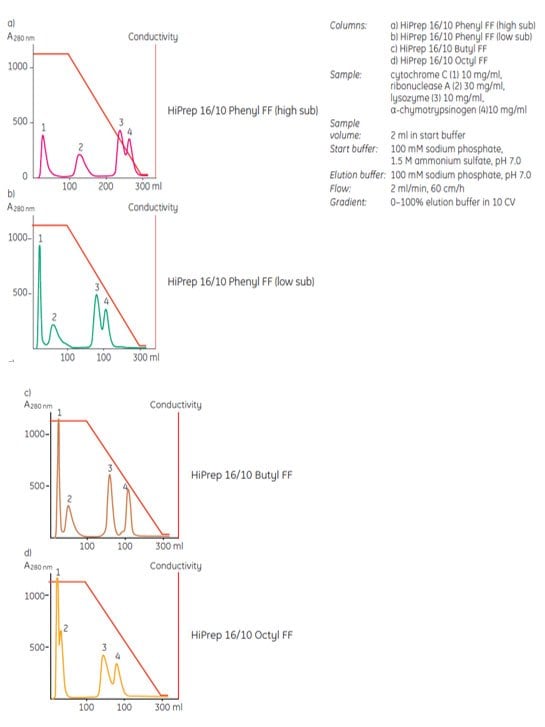
Figure 3.Different ligands and differences in ligand density influence selectivity of a HIC medium.
The binding capacity of HIC media increases with increased ligand density up to a certain level.
Simultaneously, the strength of the interaction increases, which may lead to difficulties in elution of bound components. Selecting a medium substituted with the same ligand, but at a lower ligand density, for example, Phenyl Sepharose® 6 Fast Flow (low sub) instead of Phenyl Sepharose® 6 Fast Flow (high sub) may solve the problem. Figure 14a and 14b show how the difference in ligand density between two media can influence selectivity.
The most common hydrophobic ligands are shown in Table 2. In general, HIC media fall into two groups, depending on their interactions with sample components. Straight alkyl chains (butyl, octyl, ether, isopropyl) show a "pure" hydrophobic character, while aryl ligands (phenyl) show a mixed-mode behavior in which both aromatic and hydrophobic interactions, as well as lack of charge, play a role in the final chromatographic properties.
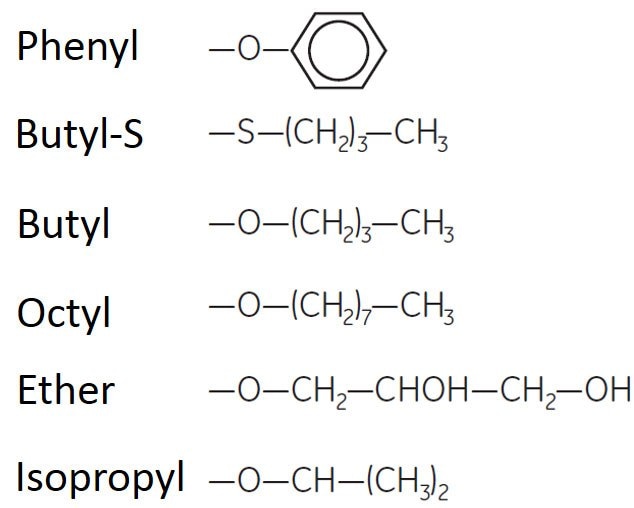
Figure 4.Ligands substituted on HIC media.
If the protein of interest does not bind under high salt conditions, use a more hydrophobic medium. If the protein of interest binds so strongly that non-polar additives are required for elution, decrease the salt concentration in the start buffer or use a less hydrophobic medium.
Selectivity and elution
Figures 5 and 6 illustrate the most common forms of HIC separation in which proteins are eluted by decreasing the salt content of a buffer using linear gradient or step elution. The UV absorbance and conductivity traces show the elution of protein peaks and the changes in salt concentration, respectively, during elution.
Buffer volumes used during sample application, elution, washing and re-equilibration are expressed in column volumes, for example 5 CV=5 mL for a column with a 1 mL bed volume.
Using column volumes to describe a separation profile facilitates method development and transfer of methods to columns of different dimensions when scaling up.
Gradient elution (Figure 5) is often used when starting with an unknown sample (as many components as possible are bound to the column and eluted differentially to see a total protein profile) and for high-resolution separation or analysis.
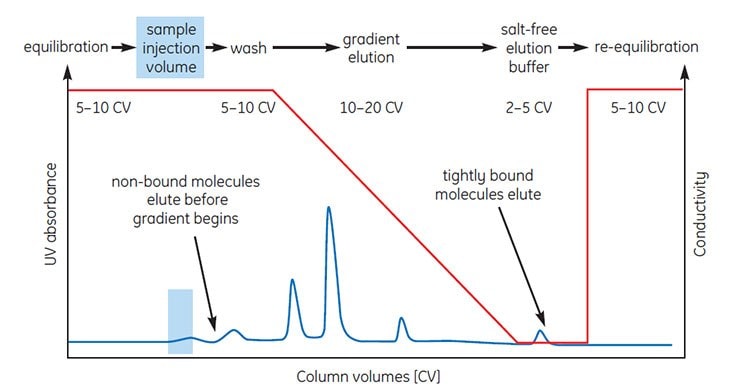
Figure 5.Typical high-resolution, HIC separation using linear gradient elution.
Step elution (Figure 6) is used in several ways. When a HIC separation has been optimized using gradient elution, changing to a step elution speeds up separation times and reduces buffer consumption while retaining the required purity level.
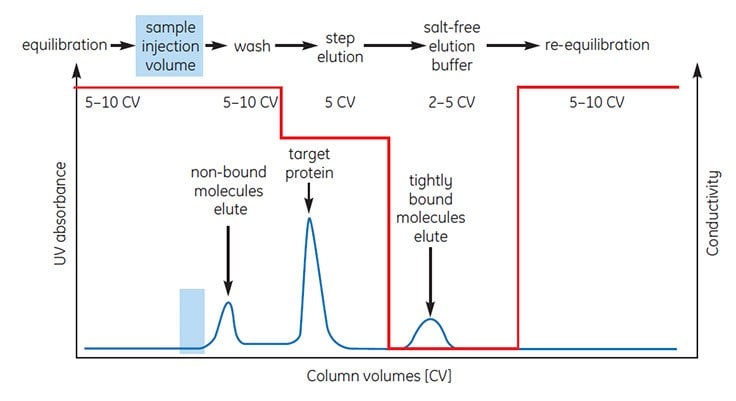
Figure 6.Typical HIC separation using step elution.
The same step elution can also be used for group separation in order to concentrate the proteins of interest and rapidly remove them from unwanted substances. The target protein(s) is eluted in an enriched, concentrated form.
Occasionally, step elution is used to remove contaminants by choosing conditions that maximize binding of the contaminants and allow the target protein(s) to pass through the column. Care must be taken to ensure that the binding capacity of the column is sufficient to bind all contaminants.
Related Products
To continue reading please sign in or create an account.
Don't Have An Account?