Performing a Purification of Antibody Fragments with Capto™ L Products
Monoclonal antibodies are typically purified using a platform approach where capture by protein A affinity chromatography has become the industry standard. However, a corresponding solution for antibody fragments has until now been lacking. One reason is the high diversity of the antibody fragments; and another is that chromatography media currently available on the market do not meet the demands for industrial-scale purification. The introduction of Capto™ L provides the foundation for a purification platform approach for this class of biomolecules.
Capto™ L products
Capto™ L is designed for capture of a wide range of antibody fragments such as Fabs, single-chain variable fragments (scFv), and domain antibodies (Dabs). Capto™ L is available in bulk sizes (5 mL to 10 L) as well as prepacked formats to support screening and optimization of binding and elution conditions. Prepacked formats include PreDictor 96-well plates and PreDictor RoboColumn units, as well as HiTrap and HiScreen prepacked columns (Figure 3.28).

Fig 3.28.Capto™ L for capture of antibody fragments and is available in bulk sizes (5 mL to 10 L) as well as prepacked formats.
In cases where Capto™ L lacks affinity for the antibody fragment of interest, there are a number of complementary affinity chromatography media available from Cytiva to enable a comprehensive capture toolkit. KappaSelect binds kappa Fabs (constant region) and Lambda FabSelect binds lambda Fabs (constant region). In addition MabSelect can be used to capture antibody fragments due to its affinity for the heavy chain subtype VH3. Figure 3.29 is a general guide for chromatography media selection.
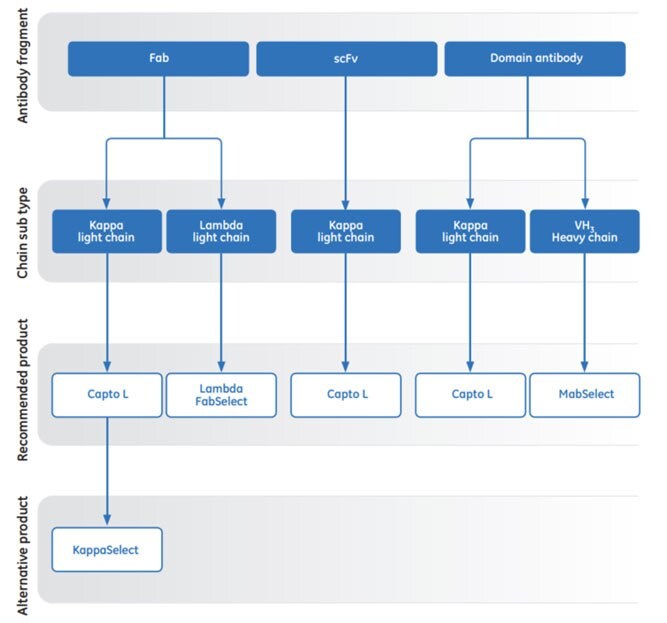
Fig 3.29.AC media for purification of antibody fragments.
Due to the high affinity binding of protein L to the variable region of the kappa light chain, Capto™ L purifies conventional Fabs as well as the smallest functional entity of antibodies, known as domain antibodies (Dabs). Figure 3.30 shows the dynamic binding capacity at 10% breakthrough (Qb10%) of Capto™ L for a number of different antibody fragments. Note that because dynamic binding capacity is normally measured in mg/mL, the molecular weight of the target molecule is an important factor to consider. Table 3.7 presents the dynamic binding capacity in relation to the molecular weight and the corresponding molar binding capacity.
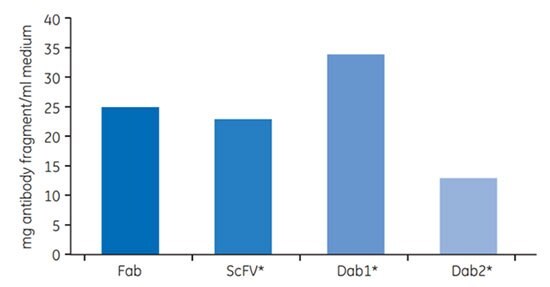
Fig 3.30.Capto™ L dynamic binding capacity at 10% breakthrough (Qb10%) of four human antibody fragments. Fab fragment kindly provided by UCB Celltech.
Antibody fragments are often expressed in microbial systems and the homogenate entering downstream purification is often crude and challenging. In the following example, a domain antibody was purified from clarified E. coli fermentation broth using Capto™ L.
Figure 3.31A shows approximately 11.4 mg Dab/mL medium loaded at pH 7.0 at a flow rate of 300 cm/h and a residence time of 4 min. A wash step followed at pH 5.0 to remove weakly bound impurities. Elution of bound material was performed with a step gradient using sodium acetate buffer, pH 3.0. Flowthrough and eluted fractions were collected and analyzed by SDS-PAGE. The elution pool contained highly enriched Dab protein, Figure 3.31B, lane 4. Product recovery was 87% and the E. coli host cell protein (HCP) levels were reduced from ~ 28 million ppm to ~ 6000 ppm. This single capture step resulted in a HCP level clearance log reduction of 3.6 and a final purity of Dab protein of 93.2%, Table 3.8.
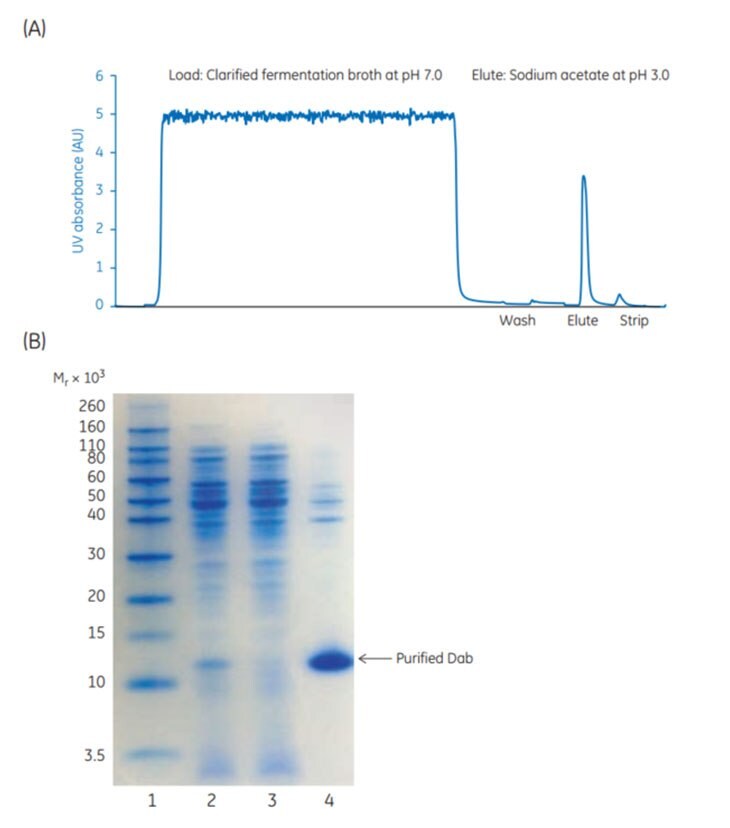
Fig 3.31.(A) Purification of Dab fragments from E. coli using Capto™ L. (B) SDS-PAGE under reducing conditions of sample load, flowthrough, and eluted fractions from the purification of Dab fragments on Capto™ L. SDS-PAGE was run under reducing conditions, Coomassie staining.
Protein G also has an affinity binding site for certain Fab regions, and consequently, Protein G AC media can in some cases be used for the purification of Fab and F(ab´)2 fragments as well. Figure 3.32 shows the purification of recombinant mouse Fab fragments, expressed in E. coli, in a single affinity purification step using Protein G Sepharose® 4 Fast Flow.
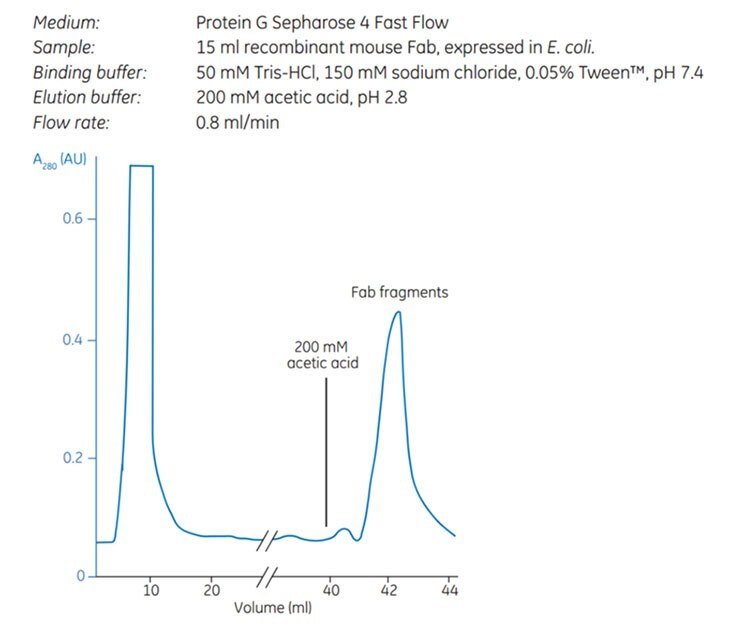
Fig. 3.32.Purification of recombinant mouse Fab fragments, expressed in E. coli using Protein G Sepharose® 4 Fast Flow.
Materials
To continue reading please sign in or create an account.
Don't Have An Account?